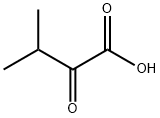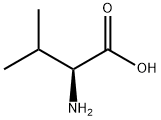
L-Valine synthesis
- Product Name:L-Valine
- CAS Number:72-18-4
- Molecular formula:C5H11NO2
- Molecular Weight:117.15
HO2CCH2CH(CH3)2 + Br2 → HO2CCHBrCH(CH3)2 + HBr
HO2CCHBrCH(CH3)2 + 2 NH3 → HO2CCH(NH2)CH(CH3)2 + NH4Br
.
640-68-6
472 suppliers
$8.00/10g

72-18-4
882 suppliers
$5.00/25g
Yield:72-18-4 98.7%
Reaction Conditions:
Stage #1: D-Val-OHwith Cupric sulfate;pyridoxal 5'-phosphate;sodium hydroxide in lithium hydroxide monohydrate at 20 - 70; for 4 h;
Stage #2: with C39H34N2O4 in chloroform-d1;
Stage #3: with hydrogenchloride in lithium hydroxide monohydrate at 20; for 2 h;
Steps:
5 Example 1: Repeat process of EECR and hydrolysis 5 times for phenylalanine
General procedure: Preparation of organic layer and aqueous layer:D-phenylalanine (0.66 g, 4.0 mmol), NaOH (0.164 g, 4.08 mmol), pyridoxal-5'-phosphate (PLP; pyridoxal-5'-phosphate) (0.011 g, 0.04 mmol), CuSO4·5H2O (0.01) g, 0.04 mmol) was dissolved in water (2 ml) to prepare an aqueous layer, (R)-Compound 1 (0.60 g, 1.0 mmol) and Aliquat-336 (0.43 g, 1.05 mmol) were dissolved in CDCl3 (1.0 mL) to prepare an organic layer.1A is a photograph of enantioselective liquid-liquid extraction using the following conventional binaphthol aldehyde derivative compound as an amino acid chiral extracting agent, and Cu2+/PLP for racemizing phenylalanine in an aqueous solution layer; This indicates that when enantioselective liquid-liquid extraction is attempted by adding a catalyst, Cu 2+ ions migrate to the organic layer and contaminate it. This makes it impossible to try liquid-liquid chiral extraction methods any more.[Conventional Binaphthol Derivative Compound].1b is a photograph of a liquid-liquid chiral extraction method using Compound 1, which is a t-butylketone derivative, as a chiral extracting agent, and a liquid-liquid chiral by adding Cu2+/PLP catalyst to racemize phenylalanine in the aqueous layer. The results of trying the extraction method are shown. Although the experiment of extracting and hydrolyzing phenylalanine and then extracting phenylalanine was repeated 5 times, it can be seen that Cu 2+ ions of the aqueous solution layer remain in the aqueous solution layer as they are. As a result of analyzing the chirality of the amino acids in the aqueous solution layer by HPLC, it can be seen that the racemization state is continuously maintained.2 shows the structural differences between the imine bond between the binaphthol derivative not containing t-butylketone-phenylalanine and compound 1-phenylalanine and when compound 1 is used as an extractant, the Cu2+ ions in the aqueous solution do not move to the organic layer. explained the reason. EECR process: After mixing the aqueous solution layer and the organic layer in a vial container, the organic layer was separated by stirring at room temperature for 6 hours. 1H NMR result of the organic layer,More than ~99% of phenylalanine obtained as imine showed very high chiral selectivity in the L-form.By reacting in a continuous process through a continuous reactor, all amino acids in the aqueous layer are optically converted to L-amino acids or all DL-amino acids to D-amino acids, or all D-amino acids or DL-amino acids to L-amino acids to obtain pure amino acids can be obtained.3A schematically illustrates the limitations of the conventional ELLE method. The ELLE method has a disadvantage in that up to 50% of the amino acids in the aqueous solution layer can be extracted into the organic layer.Figure 3b schematically illustrates the EECR process. The EECR can be extracted into the organic layer so that all of the amino acids in the aqueous solution layer have one chirality (maximum 100%).4 shows the crystal structure of Compound 1-phenylalanine obtained by recrystallizing an organic layer obtained through chiral selective extraction of phenylalanine. Hydrolysis process: The organic layer from which amino acids were extracted by the enantioselective extraction coupled with racemization (EECR) process was mixed with an aqueous hydrochloric acid solution (1.0N, 2 mL) and stirred at room temperature for 2 hours. All amino acids in the organic layer moved to the aqueous layer, and it was confirmed through 1H NMR that the organic layer returned to the state before the start of the EECR process. Repeat EECR and hydrolysis process 5 times: After the hydrolysis process, the recovered organic layer is neutralized by injecting an aqueous Na2CO3 solution, and 1 equivalent of amino acid is added to the aqueous solution layer used in the EECR process, then mixed together and stirred to perform the EECR process was performed, and a hydrolysis process was performed on the organic layer. In this way, the EECR/hydrolysis process was repeated 5 times. Recovery of optically pure phenylalanine: The EECR/hydrolysis process was repeated 5 times and the optical purity of each aqueous phenylalanine solution obtained in the hydrolysis process was measured by HPLC. As a result, the average L-type phenylalanine was 98.7%. NaOH 2.0 N aqueous solution was added to the aqueous solution to adjust the pH to 6.0, and 0.55 g of phenylalanine produced by precipitation was obtained as a solid (yield 343% based on compound 1). At this time, the imine formation rate of phenylalanine was maintained at 90% to 95% for 5 times.
References:
KR2022/21105,2022,A Location in patent:Paragraph 0108-0127; 0142-0144
![3-Butenoicacid,2-[[(1,1-dimethylethoxy)carbonyl]amino]-3-methyl-,(2S)-(9CI)](/CAS/GIF/269742-33-8.gif)
269742-33-8
9 suppliers
inquiry

72-18-4
882 suppliers
$5.00/25g
![L-Valine, N-[(2-propyn-1-yloxy)carbonyl]-](/CAS/20210305/GIF/439912-46-6.gif)
439912-46-6
0 suppliers
inquiry

72-18-4
882 suppliers
$5.00/25g
2507-77-9
0 suppliers
inquiry

72-18-4
882 suppliers
$5.00/25g