
2,6-Pyridinediamine, 3-[2-(trimethylsilyl)ethynyl]- synthesis
- Product Name:2,6-Pyridinediamine, 3-[2-(trimethylsilyl)ethynyl]-
- CAS Number:936342-41-5
- Molecular formula:C10H15N3Si
- Molecular Weight:205.33
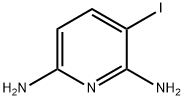
856851-34-8
56 suppliers
$120.00/1g
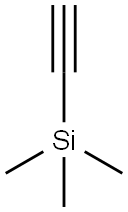
1066-54-2
472 suppliers
$9.00/10g
![2,6-Pyridinediamine, 3-[2-(trimethylsilyl)ethynyl]-](/CAS/20210111/GIF/936342-41-5.gif)
936342-41-5
2 suppliers
inquiry
Yield:936342-41-5 60%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;copper(l) iodide;tetrakis(triphenylphosphine) palladium(0) in 1-methyl-pyrrolidin-2-one at 20; for 0.5 h;Product distribution / selectivity;
Steps:
13.1.2
Manufacturing Example 13-1-2 3-Trimethylsilanylethynyl-pyridin-2,6-diamine; To a mixture of 3-iodo-pyridin-2,6-diamine (20.0 g, 85.2 mmol) described in Manufacturing Example 13-1-1, trimethylsilyl acetylene (24.2 mL, 170 mmol), copper (I) iodide (3.25 g, 17.0 mmol) N,N-diisopropylethylamine (19.1 g, 148 mmol) and N-methylpyrrolidinone (286 mL) was added tetrakis(triphenylphosphine)palladium (0) (9.81 g, 8.52 mmol) under argon atmosphere, which was stirred for 30 minutes at room temperature. The reaction solution was partitioned into water and ethyl acetate. The ethyl acetate layer was washed with water 4 times and dried over sodium sulfate, and the solvent was evaporated under a reduced pressure. The residue was purified by NH silica gel chromatography (heptane:ethyl acetate=4:1 then 1:1). The solids obtained by concentrating the eluate under a reduced pressure were washed with heptane containing a small amount of ethyl acetate to obtain the title compound (10.5 g, 60.0%).1H-NMR Spectrum (DMSO-d6) δ (ppm): 0.20 (9H, s), 5.53 (2H, brs), 5.66 (1H, d, J=8.0 Hz), 5.95 (2H, brs), 7.11 (1H, d, J=8.0 Hz).
References:
US2009/82403,2009,A1 Location in patent:Page/Page column 68