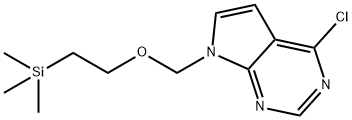
4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE synthesis
- Product Name:4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE
- CAS Number:941685-26-3
- Molecular formula:C12H18ClN3OSi
- Molecular Weight:283.83
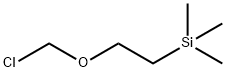
76513-69-4
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
![4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/941685-26-3.gif)
941685-26-3
Sodium hydride (340 mg, 60% dispersed in mineral oil) was added in two portions to a solution of 4-chloropyrrolo[2,3-d]pyrimidine (3a) (1.0 g, 6.5 mmol) in anhydrous DMF (15 mL) under the cooling conditions of an ice-salt bath, keeping the reaction temperature at 0 °C. The reaction temperature was controlled not to exceed 10 °C under nitrogen protection with continuous stirring for 1 hour. Subsequently, 2-(trimethylsilyl)ethoxymethyl chloride (SEMCl) (1.4 g, 8.5 mmol) was slowly added via syringe while ensuring that the temperature did not exceed 10 °C. The reaction mixture was gradually warmed to room temperature and stirring was continued overnight. After completion of the reaction, the reaction was quenched with water, extracted with ethyl acetate (EA) and the organic phase was dried over anhydrous sodium sulfate and concentrated. Purification by preparative fast chromatography (eluent: petroleum ether/ethyl acetate = 19:1) afforded 4-chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (3b) (1.834 g, oily product) in 97% yield. Mass spectrum (ESI, m/z): 284 [M + H]+.

76513-69-4
358 suppliers
$6.00/1g
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
840 suppliers
$6.00/1g
![4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/941685-26-3.gif)
941685-26-3
177 suppliers
$59.00/250mg
Yield:941685-26-3 100%
Reaction Conditions:
Stage #1: 4-chloro-1H-pyrrolo[2,3-d]pyrimidinewith sodium hydride in N,N-dimethyl-formamide at 20; for 1 h;Cooling with ice;
Stage #2: (2-trimethylethylsilylethoxy)methyl chloride in N,N-dimethyl-formamide;Cooling with ice;
Steps:
1.D Step D: 4-chloro-7-(2-(trimethylsilyl)ethoxy)methyl-7H-pyrrolo[2,3-d]pyrimidine
Under ice-cooling with stirring, 38.4g (250.4mmol, 1.0eq.) 4-chloro-7H-pyrrolo[2,3-d]pyrimidine is dissolved in 200mL dry DMF. 13g (305mmol, 1.2eq) 57% content of NaH was added. The reaction was stirred at room temperature for 1 hour. 50.9g SEMCl was added dropwise under ice-cooling (305mmol, 1.2eq.). After complete addition, the ice bath the reaction was stirred for 1 hour, quenched with water, extracted with ethyl acetate, combined organic phases were washed with brine, dried over sodium sulfate, filtered and concentrated in vacuo, column chromatography on a silica column to give the title compound (71g , yield = 100%).
References:
CN109867676,2019,A Location in patent:Paragraph 0109-0111
![Pyrrolo[2,3-d]pyrimidin-4-ol](/CAS/GIF/3680-71-5.gif)
3680-71-5
413 suppliers
$6.00/1g
![4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE](/CAS/GIF/941685-26-3.gif)
941685-26-3
177 suppliers
$59.00/250mg