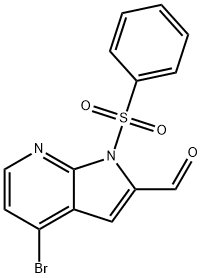
4-Bromo-1-phenylsulfonyl-7-azaindole-2-carboxyaldehyde synthesis
- Product Name:4-Bromo-1-phenylsulfonyl-7-azaindole-2-carboxyaldehyde
- CAS Number:942920-59-4
- Molecular formula:C14H9BrN2O3S
- Molecular Weight:365.2
![4-BROMO-1-(PHENYLSULFONYL)-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/889939-25-7.gif)
889939-25-7
96 suppliers
$38.00/250mg
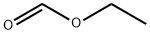
109-94-4
507 suppliers
$10.00/10g

942920-59-4
34 suppliers
$395.00/250mg
Yield:942920-59-4 3.05 g
Reaction Conditions:
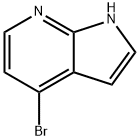
Stage #1: 1-(benzenesulfonyl)-4-bromo-1H-pyrrolo[2,3-b]pyridinewith n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2: formic acid ethyl ester in tetrahydrofuran at -78; for 0.25 h;Inert atmosphere;
Steps:
b b. 4-Bromo-1 -(phenylsulfonyl)-1 H-pyrrolo[2,3-b]pyridine-2-carbaldehyde
A solution of diisopropylamine (1.6 mL, 11.57 mmol) was dissolved in THF (15 mLand cooled to -78°C under argon. n-Butyllihium (0.88 mL, 10.68 mmol) was added dropwise and afterstirring 5 minutes at -78°C, the mixture was allowed to warm up to 0°C stirred at 0°C for 5 minutes and then cooled again to -78°C. A solution of 4-bromo-1-(phenylsulfonyl)-1H- pyrrolo[2,3-b]pyridine (Intermediate 122a, 3 g, 8.90 mmol) in THF (15 mL) added dropwise with stirring. The mixture was stirred at -78°C for 30 minutes then ethyl formate (1.4 mL, 17.79 mmol) was added and the mixture was stirred at -78°C for 15 minutes and subsequentlyquenched with saturated ammonium chloride solution. The reaction mixture was diluted with ethyl acetate/i-hexane and the organic phase was dried over MgSO4 and filtered. The filtrate was evaporated to dryness to yield the title compound as a yellow solid (3.05 g).1H NMR (400 MHz, ODd3) 10.64 (1H, 5), 8.40 (1H, d, J=5.2 Hz), 8.19-8.16 (2H, m), 7.65- 7.60 (1 H, m), 7.54 - 7.50 (2H, m), 7.47 - 7.43 (2H, m).
References:
WO2019/58132,2019,A1 Location in patent:Page/Page column 109; 110
98-09-9
364 suppliers
$12.00/25g

942920-59-4
34 suppliers
$395.00/250mg
348640-06-2
337 suppliers
$6.00/250mg

942920-59-4
34 suppliers
$395.00/250mg