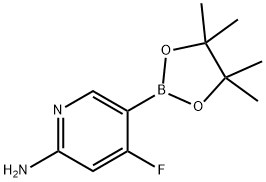
6-fluoro-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-aMine synthesis
- Product Name:6-fluoro-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-aMine
- CAS Number:944401-71-2
- Molecular formula:C11H16BFN2O2
- Molecular Weight:238.07
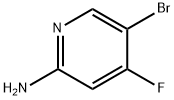
944401-69-8
104 suppliers
$45.00/50mg

73183-34-3
587 suppliers
$6.00/5g

944401-71-2
24 suppliers
inquiry
Yield:944401-71-2 100%
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane at 110; for 2 h;Sealed vessel;
Steps:
10; 13
0275] In a sealable Pyrex pressure vessel, a mixture of 5-bromo-4-fluoropyridin-2-amine (25 mg, 0.13 nxmol), 4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)-l,3,2-dioxaborolane (40 mg, 0.16 mmol), potassium acetate (51 mg, 0.52 mmol) and dichloro[l,l'-bis(diphenylphosphino)ferrocene]palladium(II)- dichloromethane adduct (16 mg, 0.019 mmol) was suspended in dioxane (1.7 mL) under argon. The pressure vessel was sealed and the reaction mixture was stirred at 110 0C for 2 hours. After the reaction was complete as judged by LCMS, the reaction mixture was cooled to room temperature and the 4-fluoro-5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)pyridin-2 -amine was used in subsequent reactions without further purification, assuming a quantitative yield (0.13 mmol). LC/MS (m/z): 157.0 (MHf^ of the boronic acid formed by product hydrolysis on LC), Rt 0.34 minutes.
References:
WO2007/84786,2007,A1 Location in patent:Page/Page column 102-103; 106; 108
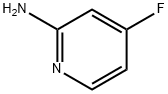
944401-77-8
225 suppliers
$17.00/1g

944401-71-2
24 suppliers
inquiry