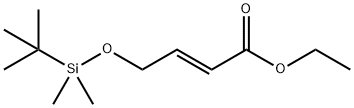
(E)-4-[[(1,1-DiMethylethyl)diMethylsilyl]oxy]-2-butenoic Acid Ethyl Ester synthesis
- Product Name:(E)-4-[[(1,1-DiMethylethyl)diMethylsilyl]oxy]-2-butenoic Acid Ethyl Ester
- CAS Number:94844-37-8
- Molecular formula:C12H24O3Si
- Molecular Weight:244.4
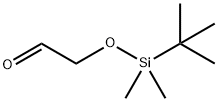
102191-92-4
139 suppliers
$10.00/250mg
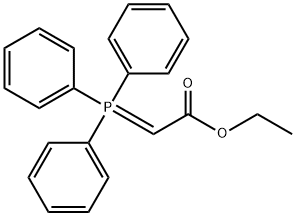
1099-45-2
358 suppliers
$6.00/5g
![(E)-4-[[(1,1-DiMethylethyl)diMethylsilyl]oxy]-2-butenoic Acid Ethyl Ester](/CAS/20150408/GIF/94844-37-8.gif)
94844-37-8
6 suppliers
inquiry
Yield:94844-37-8 98%
Reaction Conditions:
in toluene at 20 - 50; for 5 h;Inert atmosphere;
Steps:
3.3A
(E)-Ethyl 4-(tert-butyldimethylsilyloxy)but-2-enoate (3A) 2-(Tert-butyldimethylsilyloxy)acetaldehyde (10.0 g, 57.3 mmol) was added to a solution of (2-ethoxy-2-oxoethyl)triphenylphosphorane (20.0 g, 57.4 mmol) in 150 mL toluene, the mixture was stirred at RT overnight and then heated to 50° C. for 5 hrs. Solvents were removed and the residue was suspended in 400 mL of hexane. The solution was filtered, the filtrate was concentrated to give the product 3A as a colorless liquid 13.7 g (98%). 1H NMR (400 MHz, CDCl3) δ ppm 0.08 (s, 6H), 0.92 (s, 9H), 1.30 (t, 3H, 6 Hz), 4.21 (q, 2H, 6 Hz), 4.34 (dt, 2H), 6.09 (dt, 1H), 7.00 (dt, 1H); ESI-MS:m/z 245.3 (M+H)+, HPLC retention time: T=2.737 min (analytical-3).
References:
US8129538,2012,B1 Location in patent:Page/Page column 88
10080-68-9
16 suppliers
$504.31/5MG

18162-48-6
649 suppliers
$9.00/5g
![(E)-4-[[(1,1-DiMethylethyl)diMethylsilyl]oxy]-2-butenoic Acid Ethyl Ester](/CAS/20150408/GIF/94844-37-8.gif)
94844-37-8
6 suppliers
inquiry
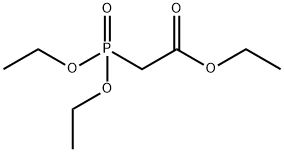
867-13-0
449 suppliers
$10.00/1g

102191-92-4
139 suppliers
$10.00/250mg
![(E)-4-[[(1,1-DiMethylethyl)diMethylsilyl]oxy]-2-butenoic Acid Ethyl Ester](/CAS/20150408/GIF/94844-37-8.gif)
94844-37-8
6 suppliers
inquiry
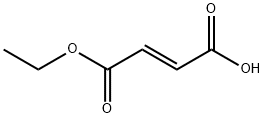
2459-05-4
238 suppliers
$9.00/25g
![(E)-4-[[(1,1-DiMethylethyl)diMethylsilyl]oxy]-2-butenoic Acid Ethyl Ester](/CAS/20150408/GIF/94844-37-8.gif)
94844-37-8
6 suppliers
inquiry