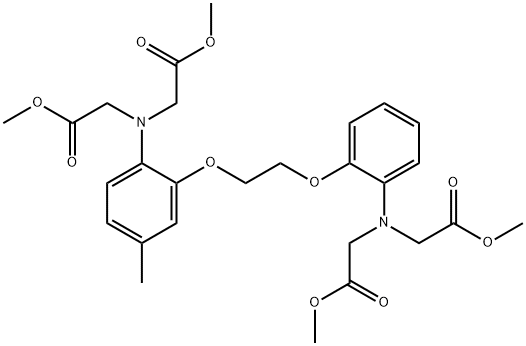
5-Methyl-bis-(2-aminophenoxymethylene)-N,N,NNtetraacetate Methyl Ester synthesis
- Product Name:5-Methyl-bis-(2-aminophenoxymethylene)-N,N,NNtetraacetate Methyl Ester
- CAS Number:96315-10-5
- Molecular formula:C27H34N2O10
- Molecular Weight:546.57
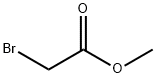
96-32-2
346 suppliers
$15.00/5g
![2-[2-(2-Aminophenoxy)ethoxy]-4-methyl-benzenamine](/CAS/GIF/96331-95-2.gif)
96331-95-2
22 suppliers
$80.00/10mg

96315-10-5
12 suppliers
$110.00/10mg
Yield:96315-10-5 23%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile at 80;
Steps:
1.4 4) Synthesis of 5-methyl BAPTA tetramethyl ester
2-[2-(2-aminophenyl)ethoxy]-4-methylbenzene amine (724 mg, 2.81 mmol), methyl bromoacetate (1.54 mL, 16.7 mmol), and DIEA (5.8 mL, 33.3 mmol) were dissolved in MeCN (20 mL) and stirred overnight at 80° C. AcOEt (20 mL) was added; after removing the salt by filtration, the solvent was removed under reduced pressure. The residue was purified by silica gel column chromatography (EtOAc/n-hexane=), and 5-methyl BAPTA tetramethyl ester was obtained (362 mg, 0.662 mmol, yield 23%). (0223) 1H NMR (400 MHz, CDCl2): δ=2.26 (s, 3H), 3.56 (s, 6H), 3.58 (s, 6H), 4.12 (s, 4H), 4.16 (s, 4H), 4.27 (s, 4H), 6.67 (d, J=4.9 Hz, 2H), 6.74 (dd, J=4.9 Hz, 3.4 Hz, 1H), 6.81-6.83 (m, 1H), 6.85-6.89 (m, 2H), 6.90-6.93 (m, 1H). 13C NMR (100 MHz, CDCl3): δ=20.9, 51.5, 51.6, 53.3, 53.4, 67.0, 67.1, 113.2, 114.1, 119.0, 119.2, 121.5, 121.7, 122.2, 132.1, 136.8, 139.2, 150.3, 150.4, 172.0, 172.0.
References:
US2020/87326,2020,A1 Location in patent:Paragraph 0222-0223
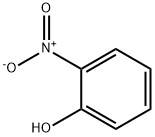
88-75-5
37 suppliers
$15.00/5mg

96315-10-5
12 suppliers
$110.00/10mg
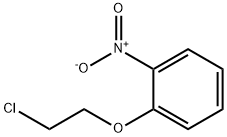
102236-25-9
16 suppliers
inquiry

96315-10-5
12 suppliers
$110.00/10mg
![4-METHYL-1-NITRO-2-[2-(2-NITROPHENOXY)ETHOXY]-BENZENE](/CAS/GIF/96315-08-1.gif)
96315-08-1
9 suppliers
inquiry

96315-10-5
12 suppliers
$110.00/10mg