
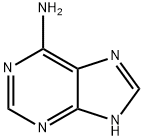
9H-Purin-6-amine, 9-propyl- synthesis
- Product Name:9H-Purin-6-amine, 9-propyl-
- CAS Number:707-98-2
- Molecular formula:C8H11N5
- Molecular Weight:177.21
Yield:707-98-2 40%
Reaction Conditions:
Stage #1: adeninewith sodium hydride in N,N-dimethyl-formamide;paraffin oil; for 0.5 h;Inert atmosphere;
Stage #2: propyl bromide in N,N-dimethyl-formamide;paraffin oil;Inert atmosphere;
Steps:
Synthesis of 9-propyladenine (9-PA)
Synthesis of 9-PA was carried out by a previously reported procedure [5b]. In brief, adenine (2.0g, 14.8mmol) was dissolved in DMF (10 mL), followed by addition of NaH (0.71 g as 60% in paraffin, 17.7mmol) and stir under nitrogen atmosphere for 30 min. After this n-propyl bromide (1.33 mL, 14.0 mmol) is added and stir overnight under nitrogen atmosphere. After this time, DMF is evaporated under high vacuum and compound is separated by column chromatography (1.05g, 40% yield). FABMS: (M++1)=178; M.P.=167°C. 1H NMR: (400MHz, CDCl3, 22°C, TMS) δ (ppm) 0.88-0.92 (t, 3H), 1.84-1.91 (m, 2H), 4.09-4.12 (t, 2H), 6.14 (s, 2H, D2O exchange), 7.75 (s, 1H), 8.30 (s, 1H). 13C NMR (100MHz, DMSO-d6, 20.9°C, TMS); δ (ppm) 10.94, 22.78, 44.51, 118.76, 140.93, 149.58, 152.38, 155.97. Anal. Calc for C8H11N5; C, 54.22; H, 6.26; N, 39.52; found C, 54.17; H, 6.22; N, 39.58.
References:
Mishra, Ashutosh Kumar;Prajapati, Rajneesh Kumar;Verma, Sandeep [Polyhedron,2013,vol. 52,p. 1385 - 1390]
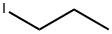
107-08-4
260 suppliers
$14.00/5g
134461-75-9
1 suppliers
inquiry

41785-15-3
0 suppliers
inquiry

707-98-2
9 suppliers
inquiry

106-94-5
583 suppliers
$10.00/5ml
73-24-5
885 suppliers
$6.00/5g

80681-19-2
5 suppliers
inquiry

707-98-2
9 suppliers
inquiry