9H-Pyrimido[4,5-b]indole synthesis
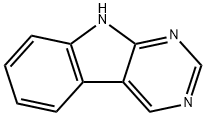
- Product Name:9H-Pyrimido[4,5-b]indole
- CAS Number:5059-31-4
- Molecular formula:C10H7N3
- Molecular Weight:169.18
Yield: 37.9%
Reaction Conditions:
with tris(dibenzylideneacetone)dipalladium(0) chloroform complex;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene;sodium t-butanolate in 1,4-dioxane at 110; for 24 h;Inert atmosphere;
Steps:
9H-Pyrimido[4,5-b]indole (Intermediate S)
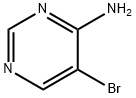
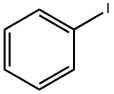
A mixture of 5-bromopyrimidin-4-amine (1 g, 5.78 mmol), iodobenzene (1.18 g, 5.78 mmol), xantphos (334 mg, 0.578 mmol), sodium tert-butoxide (1.66 g, 17.34 mmol) and tris(dibenzylideneacetone)dipalladium (0) (0.52 g, 0.58 mmol) in dioxane was degassed with nitrogen, heated to 110° C. and stirred for 24 hours. The reaction was cooled to r.t, poured into water and extracted with EtOAc (3×100 mL). The combined organic layer was washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum. The residue was purified by flash chromatography (EtOAc/DCM=80%) to give 9H-pyrimido[4,5-b]indole (370 mg, 37.9% yield) as a yellow solid. LC/MS (ESI, m/z): [M+1]+=170.0.
References:
Kymera Therapeutics, Inc.;Ji, Nan;Kluge, Arthur F.;Weiss, Matthew M.;Zhang, Yi US2020/10468, 2020, A1 Location in patent:Paragraph 0762; 0763
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![9H-Pyrimido[4,5-b]indole](/CAS/20200611/GIF/5059-31-4.gif)
5059-31-4
1 suppliers
inquiry
27878-37-1
40 suppliers
$624.00/1g
![9H-Pyrimido[4,5-b]indole](/CAS/20200611/GIF/5059-31-4.gif)
5059-31-4
1 suppliers
inquiry
![3H-1,2,3-Triazolo[4,5-d]pyrimidine, 3-phenyl-](/CAS/20210305/GIF/73112-02-4.gif)
73112-02-4
0 suppliers
inquiry
71-43-2
671 suppliers
$11.19/25ML
![9H-Pyrimido[4,5-b]indole](/CAS/20200611/GIF/5059-31-4.gif)
5059-31-4
1 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry