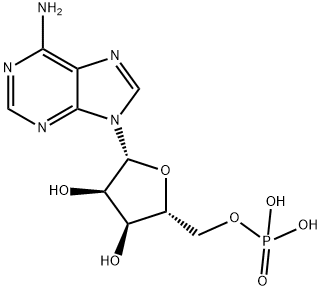
Adenosine 5'-monophosphate synthesis
- Product Name:Adenosine 5'-monophosphate
- CAS Number:61-19-8
- Molecular formula:C10H14N5O7P
- Molecular Weight:347.22
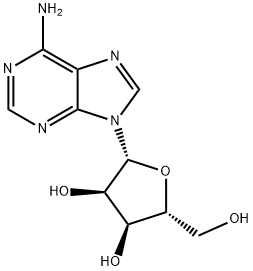
58-61-7
925 suppliers
$9.00/1g

61-19-8
530 suppliers
$5.00/1g
Yield:61-19-8 27%
Reaction Conditions:
with trichlorophosphate in lithium hydroxide monohydrate; for 0.5 h;Solvent;
Steps:
5 EXAMPLE 5 Synthetic preparation of adenosyl monophosphate
Adenosine (1000 mg, 3.74 mmol, 1.0 eq) was added to a ceramic mortar and phosphoryltrichloride (1.4 ml, 14.97 mmol, 4.0 eq), followed by 2 eq of water, was added and the mixturehand-grinded using a ceramic pestle for 30 minutes. ‘H NIVIR analysis showed 40% conversion to the desired product. Cl 8 biotage chromatography using an eluent of 100% water yielded the desired product in 27% isolated yields and recovery of the unreacted adenosine. ‘H NIVIR (400MHz, D20) ? ppm 8.50 (1H, s, Ar), 8.13 (1H, s, Ar), 6.01 (1H, m, J= 6.0 Hz), 4.42-4.37 (1H, m), 4.28-4.22 (1H, m), 3.87 (2H, t, J= 3.5 Hz). 31P NIVIR (162 MHz, D20) ? ppm 3.78.
References:
WO2016/196941,2016,A1 Location in patent:Paragraph 0251; 0252; 0253
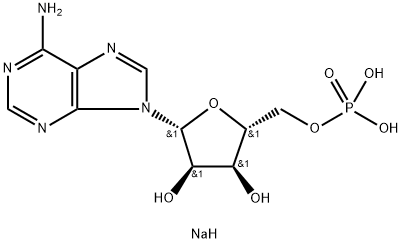
4578-31-8
411 suppliers
$5.00/5g

61-19-8
530 suppliers
$5.00/1g
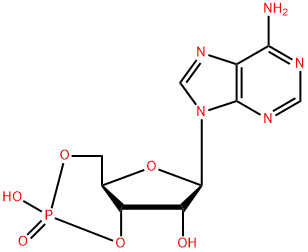
60-92-4
515 suppliers
$7.00/100mg

61-19-8
530 suppliers
$5.00/1g
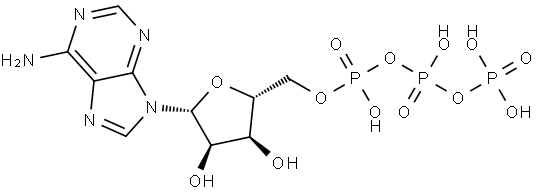
56-65-5
322 suppliers
inquiry

61-19-8
530 suppliers
$5.00/1g
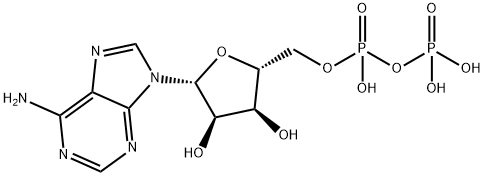
58-64-0
268 suppliers
$11.00/250mg

61-19-8
530 suppliers
$5.00/1g