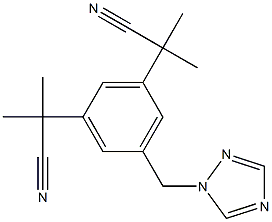
Anastrozole synthesis
- Product Name:Anastrozole
- CAS Number:120511-73-1
- Molecular formula:C17H19N5
- Molecular Weight:293.37
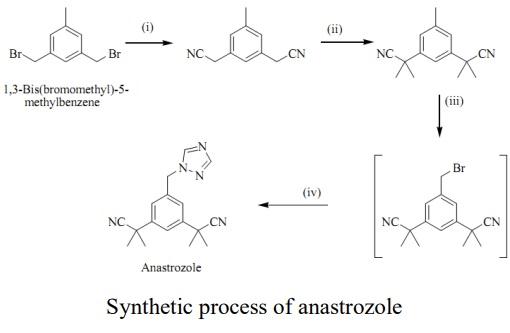
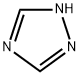
288-88-0
806 suppliers
$10.00/5g
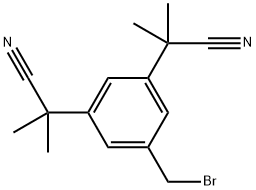
120511-84-4
217 suppliers
inquiry

120511-73-1
636 suppliers
$45.00/10mg
Yield:120511-73-1 100%
Reaction Conditions:
with potassium tert-butylate in toluene at 9 - 21; for 4.5 h;
Steps:
1.E Example 1 and variations thereof: Preparation of compound of formula II' (anastrozole) by using toluene as the solvent and a phase transfer catalyst
A) Employing toluene in a ratio of approx. 9 : 1 (v/m) relative to compound of formula I' A solution of I' (Z = Br, 328.9 g, 1.0 mol) in toluene (1.32 L) was added to a stirred mixture of K2CO3 (179.7 g, 1.3 eq.), 1,2,4-triazole (72.5 g, 1.05 eq.), PEG 600 (30.0 g, 0.05 eq.), and toluene (1.65 L) at 40 - 45 °C. After stirring for 3 h at 44 - 45 °C, the mixture (II' : III' = 8:1, calcd.) was filtered and the residue (III', approx. 41 g, 14 %, purity: 81.2 %), was washed with warm toluene (0.5 L). The filter residue was washed with water and dissolved in warm methanol (500 mL). The resulting solution was evaporated to dryness to give 40.9 g (13.9 %) of isomer III' (81.2 %, HPLC). The filtrate was washed with water, obtaining 3.56 L of a solution of compound II'. HPLC: 89.4 % of compound II', 3.5 % of isomer III' (3.5 %), corresponding to an isomeric ratio of approx. 26:1 (II' : III'). Yield: (91.3 %, calcd.).; E) Employing 2.2 eq of KtOBu [0070] Similar to example 1, item A) but stirring at 9 - 21 °C for 4.5 h. Yield: quantitative. HPLC: 75.16 % of compound II', 3.05 % of isomer III', corresponding to an isomeric ratio of approx. 25:1 (II' : III').
References:
EP2343278,2011,A1 Location in patent:Paragraph 0070

288-88-0
806 suppliers
$10.00/5g
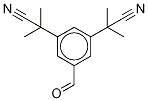
120511-89-9
16 suppliers
inquiry

120511-73-1
636 suppliers
$45.00/10mg

120511-84-4
217 suppliers
inquiry
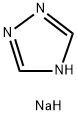
41253-21-8
283 suppliers
$5.00/5g

120511-73-1
636 suppliers
$45.00/10mg
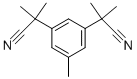
120511-72-0
278 suppliers
$11.00/250mg

41253-21-8
283 suppliers
$5.00/5g

120511-73-1
636 suppliers
$45.00/10mg
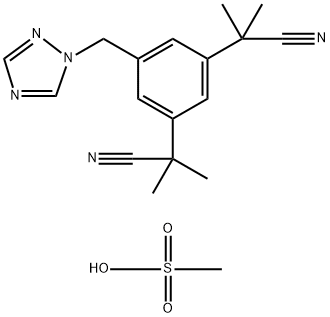
1015083-81-4
1 suppliers
inquiry

120511-73-1
636 suppliers
$45.00/10mg