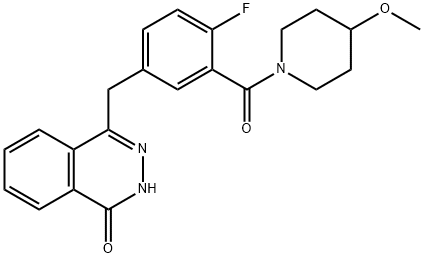
AZD2461 synthesis
- Product Name:AZD2461
- CAS Number:1174043-16-3
- Molecular formula:C22H22FN3O3
- Molecular Weight:395.43
763114-26-7
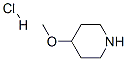
4045-25-4

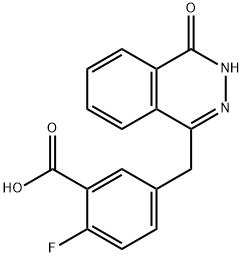
1174043-16-3
Example 1; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (231 g, 1.206 mol) was added to a solution of dichloromethane (4.7 g, 0.251 mol) containing 4-methoxypiperidine hydrochloride (183 g, 1.206 mol), 5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluorobenzoic acid (300 g, 1.005 mol), and 4-dimethylamino pyridine (30.7 g, 0.251 mol) in a solution of dichloromethane (4 L) was reacted at 23 °C. The resulting suspension was stirred at room temperature overnight. The reaction mixture was washed sequentially with 2 M hydrochloric acid (5 L) and 50% saturated sodium carbonate solution (3 L), followed by drying with anhydrous magnesium sulfate, filtration and concentration in vacuo to give the crude product. The crude product was slurried in ethyl acetate (750 mL) for 5 days, filtered and dried at 45 °C for 5 h to afford 4-[4-fluoro-3-[(4-methoxypiperidin-1-yl)carbonyl]benzyl]phthalazin-1(2H)-one (Compound 1) (290 g, 72.9% yield).1H NMR (400.132 MHz, DMSO) δ 1.26-1.35 ( 1H, m), 1.40-1.49 (1H, m), 1.69-1.73 (1H, m), 1.84-1.89 (1H, m), 2.99-3.07 (1H, m), 3.25 (3H, s), 3.27-3.34 (2H, m), 3.39-3.44 (1H, m), 3.86-3.95 (1H, m), 4.33 (2H, s). 4.33 (2H, s), 7.19-7.24 (1H, m), 7.33-7.35 (1H, m), 7.39-7.43 (1H, m), 7.81-7.91 (2H, m), 7.97 (1H, d), 8.27 (1H, d), 12.57 (1H, s); m/z (ES+) (M + H)+ = 396.31 ; HPLC tR = 1.90 min. Figure 1 illustrates the powder XRD pattern of the product showing it in crystalline form. Figure 2 illustrates the DSC analysis of the product.

763114-26-7
299 suppliers
$6.00/250mg
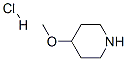
4045-25-4
112 suppliers
$7.00/250mg

1174043-16-3
116 suppliers
$35.00/1mg
Yield: 72.9%
Reaction Conditions:
with dmap;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 20 - 23;Product distribution / selectivity;
Steps:
1
Example 1; 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (231 g, 1206.97 mmol) was added to 4-methoxypiperidine hydrochloride (183 g, 1206.97 mmol), 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid (300 g, 1005.80 mmol) and 4-dimethylaminopyridine (30.7 g, 251.45 mmol) in DCM (4 L) at 23° C. The resulting suspension was stirred at room temperature over night. The reaction mixture was washed with 2M HCl (5 L) and 50% saturated sodium carbonate (3 L) before being dried over MgSO4, filtered and reduced in-vacuo to give the crude product. This was then slurried in 750 ml of ethyl acetate for 5 days, and then filtered and dried at 45° C. for 5 hours to afford 4-(4-fluoro-3-(4-methoxypiperidine-1-carbonyl)benzyl)phthalazin-1(2H)-one (compound 1)(290 g, 72.9%).1H NMR (400.132 MHz, DMSO) δ 1.26-1.35 (1H, m), 1.40-1.49 (1H, m), 1.69-1.73 (1H, m), 1.84-1.89 (1H, m), 2.99-3.07 (1H, m), 3.25 (3H, s), 3.27-3.34 (2H, m), 3.39-3.44 (1H, m), 3.86-3.95 (1H, m), 4.33 (2H, s), 7.19-7.24 (1H, m), 7.33-7.35 (1H, m), 7.39-7.43 (1H, m), 7.81-7.91 (2H, m), 7.97 (1H, d), 8.27 (1H, d), 12.57 (1H, s); m/z (ES+) (M+H)+=396.31; HPLC tR=1.90 min.FIG. 1 shows the powder XRD pattern of the material produced, which is in Form C.FIG. 2 shows the DSC analysis of the material produced.
References:
ASTRAZENECA AB US2011/15393, 2011, A1 Location in patent:Page/Page column 7

763114-26-7
299 suppliers
$6.00/250mg
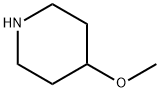
4045-24-3
148 suppliers
$38.00/1g

1174043-16-3
116 suppliers
$35.00/1mg