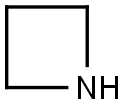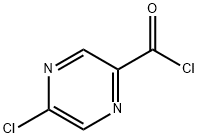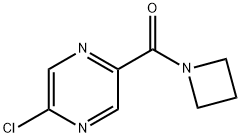
Azetidin-1-yl(5-chloropyrazin-2-yl)Methanone synthesis
- Product Name:Azetidin-1-yl(5-chloropyrazin-2-yl)Methanone
- CAS Number:915948-98-0
- Molecular formula:C8H8ClN3O
- Molecular Weight:197.62
Yield:915948-98-0 82%
Reaction Conditions:
Stage #1: 5-chloropyrazinoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 2 h;
Stage #2: azetidinewith triethylamine in dichloromethane at 20; for 72 h;
Steps:
32
Intermediate 32: Azetidin-1-yl-(5-chloropyrazin-2-yl)methanone Oxalyl chloride (1.55 mL, 17.5 mmol), followed by DMF (2 drops), was added to a mixture of 5-chloropyrazine-2-carboxylic acid (CAS no. 36070-80-1, Intermediate 33) (2.31 g, 14.6 mmol) in DCM (40 mL). The reaction was stirred at RT for 2 hours after which time the volatiles were removed under reduced pressure. The residue was taken up DCM (40 mL) and azetidine (1.08 mL, 16.03 mmol) and triethylamine (4.46 mL, 32.06 mmol) added. The mixture was stirred at RT for 72 hours. The volatiles were removed under reduced pressure and ethyl acetate (100 mL) added to the residue. The organics were washed with water (100 mL), citric acid (50 mL), saturated sodium bicarbonate solution (50 mL) and brine (50 mL), dried (MgSO4), filtered and the solvent removed under reduced pressure. The residue was purified by flash chromatography, eluding with a gradient of 50-100% ethyl acetate in isohexane, to afford the product (2.38 g, 82%). 1H-NMR δ (400 MHZ, CDCl3): 2.35-2.42 (2H, m), 4.26 (2H, t), 4.67 (2H, t), 8.52 (1H, d), 9.09 (1H, d); m/z 198 (M+H)+.
References:
US2008/171734,2008,A1 Location in patent:Page/Page column 44-45
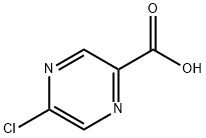
36070-80-1
279 suppliers
$9.00/1g

915948-98-0
19 suppliers
inquiry
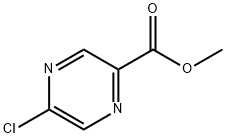
33332-25-1
246 suppliers
$8.00/1g

915948-98-0
19 suppliers
inquiry
57005-60-4
7 suppliers
inquiry

915948-98-0
19 suppliers
inquiry