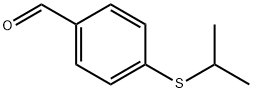
Benzaldehyde, 4-[(1-methylethyl)thio]- synthesis
- Product Name:Benzaldehyde, 4-[(1-methylethyl)thio]-
- CAS Number:84264-99-3
- Molecular formula:C10H12OS
- Molecular Weight:180.27
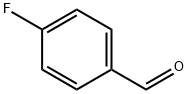
459-57-4
579 suppliers
$6.00/25g
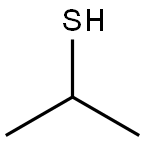
75-33-2
217 suppliers
$10.00/1g
![Benzaldehyde, 4-[(1-methylethyl)thio]-](/CAS/20180703/GIF/84264-99-3.gif)
84264-99-3
12 suppliers
$120.00/100mg
Yield:84264-99-3 87%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 100; for 16 h;
Steps:
89
To a solution of 43 (5.00 g, 40.3 mmol) in DMSO (25 mL) was added propane-2-thiol (3.38 g, 44.3 mmol) and K2C03 (11.1 g, 80.6 mmol). The mixture was stirred at 100 C for 16 h. The mixture was cooled to 15 C and and poured into ice-water (30 mL) and stirred for 20 mins. The aqueous phase wasextracted with EtOAc (20 mL x 3). The combined organic phase was washed with brine (15 mL x 2), dried over Na2SO4, filtered and concentrated in vacuum to give 44 (6.3 g, 87%) as a colorless oil. 1H NMR (400 MHz, DMSO-d6) 9.93 (s, 1 H), 7.82 (d, J = 8.4 Hz, 2H), 7.49 (d, J = 8.4 Hz, 2H), 3.79-3.72 (s, 1H), 1.31 (d, J= 7.6 Hz, 6H). To a solution of44 (3.00 g, 16.6 mmol) in THF (20 mL) and MeOH (4 mL) was added NaBH4 (755 mg, 20.0 mmol) in portions at 0 C, and then the mbdure was stirred at 15 C for2 h. The reaction mixture was quenched by water (20 mL) at 0 C, and then extracted with DCM (20 mLx 3). The combined organic layers were dried over Na2SO4, filtered and concentrated under reducedpressure to give 45 (2.99 g, 99%) as a colorless oil. 1H NMR (400 MHz, CDCI3) 7.38 (d, J = 8.0 Hz, 2H),7.295 (d, J= 8.0 Hz, 2H), 4.66 (s, 2H), 3.39-3.32 (m, 1H), 1.28 (d, J= 6.4 Hz, 6H).
References:
WO2017/195069,2017,A1 Location in patent:Page/Page column 72
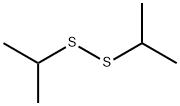
4253-89-8
154 suppliers
$45.00/250mg
1122-91-4
671 suppliers
$9.00/10g
![Benzaldehyde, 4-[(1-methylethyl)thio]-](/CAS/20180703/GIF/84264-99-3.gif)
84264-99-3
12 suppliers
$120.00/100mg