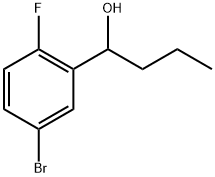
Benzenemethanol, 5-bromo-2-fluoro-α-propyl- synthesis
- Product Name:Benzenemethanol, 5-bromo-2-fluoro-α-propyl-
- CAS Number:1197943-58-0
- Molecular formula:C10H12BrFO
- Molecular Weight:247.11

106-94-5
580 suppliers
$10.00/5ml
93777-26-5
370 suppliers
$5.00/5g

1197943-58-0
1 suppliers
inquiry
Yield:1197943-58-0 92.6%
Reaction Conditions:
Stage #1: propyl bromidewith iodine;magnesium in diethyl ether at 20;
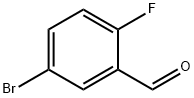
Stage #2: 5-bromo-2-fluorobenzaldehyde in diethyl ether at 20;Cooling;
Stage #3: with water;ammonium chloride in diethyl ether;
Steps:
6
Acid Preparation 6; 3-Propyl-1 H-indazole-5-carboxvlic acid; To a suspension of Mg (0.236 g, 9.83 mmol) in diethyl ether (10 mL), I2 (1 spatula) was added n-propyl bromide (0.72 g, 0.53 mL, 5.9 mmol) at room temperature. When iodine color disappeared, the remaining n-propyl bromide in ether was added to the reaction mixture and stirred at room temperature for 10 minutes. To this Grignard reagent, 5-bromo- 2-fluorobenzaldehyde (1.00 g, 4.92 mmol) in ether was added under cold conditions and stirred at room temperature for 1 hour. The reaction mixture was quenched with NH4CI solution and extracted with ether. The ether layer was dried over anhydrous Na2SO4, filtered and concentrated to afford 1 -(5-bromo-2-fluoro-phenyl)-butan-1 -ol (1.14 g, 92.6%). 1H NMR (CDCI3): δ 7.6 (m, 1 H), 7.3-7.4 (m, 1 H), 6.9 (t, 1 H), 5.0 (brs, 1 H), 2.0 (brs, 1 H), 1.6-1.7 (m, 2H), 1.3-1.5 (m, 2H), 1.0 (t, 3H).
References:
WO2009/144554,2009,A1 Location in patent:Page/Page column 44
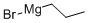
927-77-5
114 suppliers
$203.00/250g

93777-26-5
370 suppliers
$5.00/5g

1197943-58-0
1 suppliers
inquiry