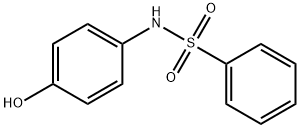
Benzenesulfonamide, N-(4-hydroxyphenyl)- synthesis
- Product Name:Benzenesulfonamide, N-(4-hydroxyphenyl)-
- CAS Number:5471-90-9
- Molecular formula:C12H11NO3S
- Molecular Weight:249.29
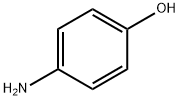
123-30-8
629 suppliers
$10.00/5g
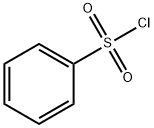
98-09-9
362 suppliers
$12.00/25g

5471-90-9
39 suppliers
$50.00/250mg
Yield:5471-90-9 77%
Reaction Conditions:
with pyridine in N,N-dimethyl-formamide at 0 - 20;Inert atmosphere;
Steps:
4.2.1. General procedure 1. Sulfonylation of para-aminophenol: preparation of N-(4-hydroxyphenyl)-benzenesulfonamide 8a (R4=SO2Ph)
General procedure: To a cooled (0 °C) solution of para-aminophenol (10,9 g, 100 mmol) in DMF (20 mL) was added pyridine (20 mL) and then dropwise (over a 10 min period) benzenesulfonyl chloride (12,8 mL, 100 mmol). The mixture was allowed to warm to rt and was stirred for one additional hour. After this time, water (150 mL) and EtOAc (100 mL) were added. After decantation, the aqueous phase was extracted with EtOAc (100 mL). The combined organic phases were washed with water (150 mL), 5% w/w aqueous solution of copper sulfate (150 mL) and brine (150 mL), dried over Na2SO4 and filtered. After removal of the solvent under reduced pressure, the crude product was purified by flash chromatography (cyclohexane/EtOAc 60:40) to afford compound 8a (19.22 g, 77%) as a white solid; mp 160 °C (lit.13 156 °C). 1H NMR (300 MHz, DMSO-d6): δ=6.59 (2H, d, J=9.0, ArH), 6.82 (2H, d, J=9.0, ArH), 7.51 (2H, m, SO2(m-ArH)), 7.59 (1H, m, SO2(p-ArH)), 7.63-7.67 (2H, m, SO2(o-ArH)), 9.31 (1H, very br s, NHSO2Ph or OH), 9.71 (1H, very br s, OH or NHSO2Ph).
References:
Baragona, Fabien;Lomberget, Thierry;Duchamp, Christian;Henriques, Natali;Lo Piccolo, Eugenio;Diana, Patrizia;Montalbano, Alessandra;Barret, Roland [Tetrahedron,2011,vol. 67,# 45,p. 8731 - 8739] Location in patent:experimental part
368-43-4
69 suppliers
$15.00/2g

123-30-8
629 suppliers
$10.00/5g

5471-90-9
39 suppliers
$50.00/250mg
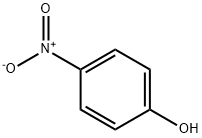
100-02-7
54 suppliers
$11.00/5G
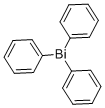
603-33-8
258 suppliers
$30.00/5g

5471-90-9
39 suppliers
$50.00/250mg
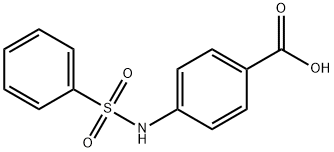
28547-16-2
49 suppliers
$25.00/250mg

5471-90-9
39 suppliers
$50.00/250mg
![Benzoyl chloride, 4-[(phenylsulfonyl)amino]-](/CAS/20210305/GIF/63421-73-8.gif)
63421-73-8
0 suppliers
inquiry

5471-90-9
39 suppliers
$50.00/250mg