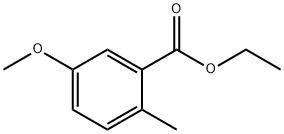
BENZOIC ACID, 5-METHOXY-2-METHYL-, ETHYL ESTER synthesis
- Product Name:BENZOIC ACID, 5-METHOXY-2-METHYL-, ETHYL ESTER
- CAS Number:855949-35-8
- Molecular formula:C11H14O3
- Molecular Weight:194.23
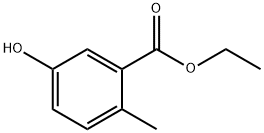
34265-55-9

74-88-4

855949-35-8
Step D: Synthesis of ethyl 5-methoxy-2-methylbenzoate as an intermediate; sodium hydride (60% dispersed in mineral oil, 1.50 g, 37.4 mmol) was suspended in N,N-dimethylformamide (50 mL) under nitrogen protection and stirred for 10 min. Subsequently, a solution of ethyl 5-hydroxy-2-methylbenzoate (5.18 g, 28.7 mmol) in N,N-dimethylformamide (100 mL) was slowly added dropwise. After the dropwise addition was completed, stirring of the reaction mixture was continued for 3 hours at room temperature. Next, iodomethane (2.69 mL, 43.1 mmol) was added and the reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the mixture was poured into water (200 mL) and extracted with ethyl acetate (200 mL). The organic phase was washed sequentially with 5% aqueous lithium chloride (200 mL), water (200 mL) and brine (100 mL), dried over anhydrous magnesium sulfate and concentrated under reduced pressure. Purification by fast column chromatography (silica gel, ethyl acetate/hexane=1:19) afforded ethyl 5-methoxy-2-methylbenzoate (4.18 g, 75% yield) as a colorless oily liquid.1H NMR (300 MHz, CDCl3) δ 7.44 (d, J=2.7 Hz, 1H), 7.14 (d, J=8.4 Hz, 1H), 6.95 ( dd, J=2.7,8.4Hz, 1H), 4.35 (q, J=7.2Hz, 2H), 3.82 (s, 3H), 2.51 (s, 3H), 1.39 (t, J=7.2Hz, 3H).

34265-55-9
9 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g

855949-35-8
24 suppliers
$5.00/100mg
Yield:855949-35-8 75%
Reaction Conditions:
Stage #1: ethyl 3-hydroxy-6-methylbenzoatewith sodium hydride in N,N-dimethyl-formamide at 20; for 3.16667 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 20;
Steps:
1.D
Step D: Synthesis of ethyl 5-methoxy-2-methylbenzoate as an intermediate; [0097] A mixture of sodium hydride (60% in mineral oil, 1.50 g, 37.4 mmol) in N,N- dimethylformamide (50 mL) was stirred for 10 min under a nitrogen atmosphere prior to dropwise addition of ethyl 5-hydroxy-2-methylbenzoate (5.18 g, 28.7 mmol) as a solution in λ/,λf-dimethylformamide (100 mL). After this time, the mixture was allowed to stir for 3 h at room temperature before iodomethane (2.69 mL, 43.1 mmol) was added and the mixture stirred overnight at room temperature. After this time, the mixture was poured into water (200 mL) and extracted with ethyl acetate (200 mL). The organics were then washed with 5% aqueous lithium chloride (200 mL), water (200 mL) and brine (100 mL), dried over magnesium sulfate and concentrated under reduced pressure. Purification by flash chromatography (silica, 1 :19 ethyl acetate/hexanes) afforded ethyl 5-methoxy-2- methylbenzoate (4.18 g, 75%) as a clear oil: 1H NMR (300 MHz, CDCl3) δ 7.44 (d, J= 2.7 Hz, IH), 7.14 (d, J= 8.4 Hz, IH), 6.95 (dd, J= 2.7, 3.0, 8.4, 8.7 Hz, IH), 4.35 (q, J= 6.9, 7.2 Hz, 2H), 3.82 (s, 3H), 2.51 (s, 3H), 1.39 (t, J= 7.2 Hz, 3H).
References:
WO2009/42907,2009,A1 Location in patent:Page/Page column 35

64-17-5
825 suppliers
$10.00/50g
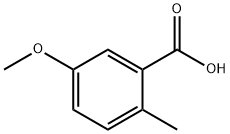
3168-59-0
122 suppliers
$15.00/1g

855949-35-8
24 suppliers
$5.00/100mg