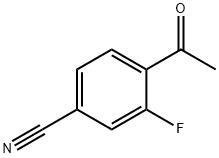
4-acetyl-3-fluorobenzonitrile synthesis
- Product Name:4-acetyl-3-fluorobenzonitrile
- CAS Number:1352144-78-5
- Molecular formula:C9H6FNO
- Molecular Weight:163.15
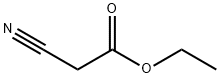
105-56-6
461 suppliers
$10.00/5ml
625446-22-2
195 suppliers
$6.00/250mg

1352144-78-5
16 suppliers
inquiry
Yield: 95.1%
Reaction Conditions:
with N,N,N,N,-tetramethylethylenediamine;palladium diacetate;sodium carbonate;1,2-bis-(diphenylphosphino)ethane;potassium iodide in N,N-dimethyl-formamide at 130; for 24 h;Inert atmosphere;
Steps:
1 Example 1:
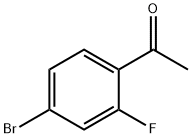
Under nitrogen protection, add 1-(4-bromo-2-fluorophenyl)ethanone (II) (2.2g, 10mmol), ethyl cyanoacetate (3.4g, 30mmol), palladium acetate (0.45 g, 2mmol), 1,2-bis(diphenylphosphine)ethane (1.2g, 3mmol), tetramethylethylenediamine (1.2g, 10mmol), sodium carbonate (3.18g, 30mmol), potassium iodide (1.7 g, 10mmol) and solvent N, N-dimethylformamide 50mL.The temperature was raised to 130°C and reacted for 24 hours.After cooling to room temperature, 300 mL of ethyl acetate was added.After stirring for 15 minutes, filter to remove insoluble materials.The organic phase was washed sequentially with saturated brine and water.Dry over anhydrous magnesium sulfate, and recover the solvent by distillation under reduced pressure. The residue obtained is recrystallized with ethyl acetate and n-hexane (volume ratio 1:1) to obtain a pale yellow solid 4-acetyl-3-fluoro-benzonitrile (III) 1.55 g, the yield was 95.1%,
References:
Suzhou Mingrui Pharmaceutical Technology Co., Ltd.;Xu Xuenong CN111517985, 2020, A Location in patent:Paragraph 0043; 0044
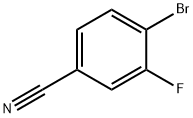
133059-44-6
236 suppliers
$5.00/1g
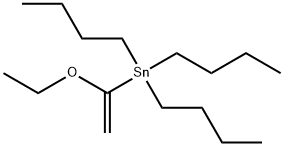
97674-02-7
233 suppliers
$15.00/1 g

1352144-78-5
16 suppliers
inquiry
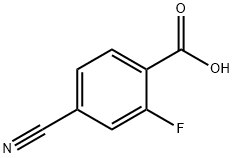
164149-28-4
179 suppliers
$8.00/1g

1352144-78-5
16 suppliers
inquiry

175596-02-8
10 suppliers
inquiry

1352144-78-5
16 suppliers
inquiry
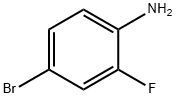
367-24-8
509 suppliers
$15.00/25 g

1352144-78-5
16 suppliers
inquiry