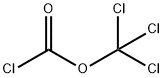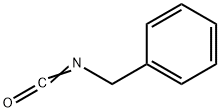
Benzyl isocyanate synthesis
- Product Name:Benzyl isocyanate
- CAS Number:3173-56-6
- Molecular formula:C8H7NO
- Molecular Weight:133.15
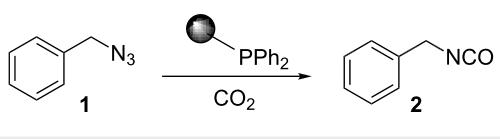
Scheme 1: Synthesis of benzyl isocyanate.
One-pot sequential synthesis of isocyanates and urea derivatives via a microwave-assisted Staudinger–aza-Wittig reaction
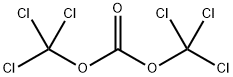
32315-10-9
441 suppliers
$10.00/1g

100-46-9
502 suppliers
$5.00/5 g

3173-56-6
239 suppliers
$21.00/1g
Yield:3173-56-6 100%
Reaction Conditions:
with triethylamine in dichloromethane
Steps:
General procedure C: preparation of N-alkyl-3-oxobenzo[d]isothiazole-2(3H)-carboxamide analogues 6a-w
General procedure: To a solution of triphosgene (2.96 g, 10 mmol) in DCM (20 ml) was added dropwise to primary amine 4 (10 mmol) in DCM (20 ml) followed by the dropwise addition of triethylamine (3 ml) in DCM (10 ml). The solvent was removed on a rotary evaporator. The resulting residue was dissolved in DCM (20 ml), and 1,2-benzisothiazol-3-one (1.51 g, 10 mmol) in THF (20 ml) was added. After the mixture was refluxed for 30 min, the solvent was removed on a rotary evaporator. The residue was dissolved in acetone (30 ml) and mixed with water (30 ml). The precipitate was collected on a funnel by vacuum filtration and washed with water-acetone (1:1,4 × 5 ml) to afford the final compound.
References:
Liu, Dazhi;Tian, Zhen;Yan, Zhihui;Wu, Lixin;Ma, Yan;Wang, Quan;Liu, Wei;Zhou, Honggang;Yang, Cheng [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 11,p. 2960 - 2967]

124-38-9
134 suppliers
$175.00/23402
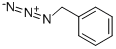
622-79-7
117 suppliers
$15.00/250mg

3173-56-6
239 suppliers
$21.00/1g

124-38-9
134 suppliers
$175.00/23402

100-46-9
502 suppliers
$5.00/5 g
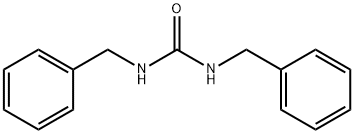
1466-67-7
59 suppliers
$62.67/0.5g

3173-56-6
239 suppliers
$21.00/1g
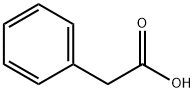
103-82-2
0 suppliers
$22.19/5g

3173-56-6
239 suppliers
$21.00/1g