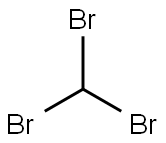
Bicyclo[5.1.0]octane, 8,8-dibromo- synthesis
- Product Name:Bicyclo[5.1.0]octane, 8,8-dibromo-
- CAS Number:7124-41-6
- Molecular formula:C8H12Br2
- Molecular Weight:267.9889
Yield:7124-41-6 90%
Reaction Conditions:
with potassium tert-butylate in pentane at -5 - 20;Inert atmosphere;
Steps:
1.2.a
3.65 g of cycloheptene 15 (38 mmol) and then 8.52 g of t-BuOK (76 mmol) and 9 ml of anhydrous pentane are introduced into a dry round-bottomed flask under argon. The solution, placed in an ice/salt bath (T<-5° C.), is vigorously stirred and then 4.9 ml of bromoform (57 mmol) are added dropwise. Once the addition is complete, the mixture is allowed to return to AT overnight, under argon and with vigorous stirring. Approximately 50 ml of water are subsequently added and the pH is neutralized with 1M HCl. The organic and aqueous phases are separated; the aqueous phase is extracted with 3×20 ml of pentane and the pentane phase is washed with 3×20 ml of water. After drying over MgSO4, the solvent is evaporated under vacuum to give an orangey-yellow oil. The product 16 is subsequently purified by filtration through silica with, as eluent, a cyclohexane/5% AcOEt mixture. A colorless oil with a total weight of 9.10 g is then obtained, equivalent to a yield of 90% (litt. 52-65% for 9,9-dibromobicyclo[6.1.0]nonane). Rf (cyclohexane 95/AcOEt 5)=0.85.1H NMR (CDCl3, 400 MHz): δ (ppm) 1.05-1.22 (m, 3H, H4-2-6); 1.34 (qq, J=1.0-7.5 Hz, 2H, H3-5); 1.68 (ddd, J=1.5-4.0-10.5 Hz, 2H, H1-7); 1.76-1.92 (m, 3H, H4'-3'-5'); 2.23 (dtq, J=14.0-6.0-1.0 Hz, 2H, H2'-6').
References:
US2011/118484,2011,A1 Location in patent:Page/Page column 5-6
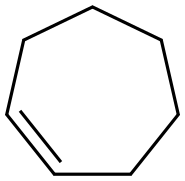
628-92-2
79 suppliers
$60.00/500mg
![Bicyclo[5.1.0]octane, 8,8-dibromo-](/CAS/20180601/GIF/7124-41-6.gif)
7124-41-6
1 suppliers
inquiry