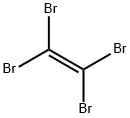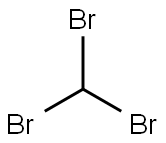
Bromoform synthesis
- Product Name:Bromoform
- CAS Number:75-25-2
- Molecular formula:CHBr3
- Molecular Weight:252.73
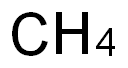
34557-54-5
0 suppliers
inquiry
74-83-9
0 suppliers
$28.30/1ML

75-25-2
341 suppliers
$16.00/50g

74-95-3
265 suppliers
$10.00/10g
Yield:-
Reaction Conditions:
with bromine at 510; under 22502.3 Torr;Gas phase;Industry scale;
Steps:
1
EXAMPLE 1Referring to Figure 1, the flow rate in methane feed line 1 to the bromination reactor 100 is 100 kg/hr, the flow rate in methane recycle line 2 is 73 kg/hr and the flow rate in the bromine feed line 3 is 1164 kg/hr. Reactor 100 is operated at 5100C and300OkPa. The conversion of methane is 50% and the selectivity to monobromomethane is 67%.Reproportionation reactor 120 is also operated at 5100C and 300OkPa. The conversion is 43% and the selectivity to monobromomethane is 100%. Coupling reactor 140 is operated at 425°C and 250OkPa. The conversion of the monobromomethane is 100% and the selectivity to BTX is 32%.Bromine generation reactor 110 is operated at 375°C and 20OkPa and the flow rate of air through feed line 36 is 554 kg/hr. The conversion and selectivity are both 100%. The flow rate in water stream 39 is 131 kg/hr. Steam cracker 270 is operated at 8400C and 10OkPa. The conversion of the monobromomethane is 84% and the selectivity to lower olefins is 60%.14 kg/hr of benzene and 23 kg/hr of p-xylene are produced. 25 kg/hr of ethylene and 12 kg/hr of propylene are produced.
References:
WO2010/42440,2010,A1 Location in patent:Page/Page column 20
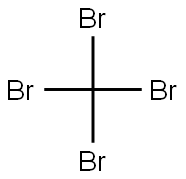
558-13-4
257 suppliers
$10.00/1g
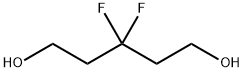
126300-39-8
10 suppliers
inquiry

75-25-2
341 suppliers
$16.00/50g
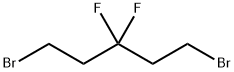
35192-43-9
5 suppliers
inquiry
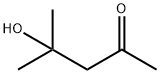
123-42-2
595 suppliers
$20.48/100ml

75-25-2
341 suppliers
$16.00/50g
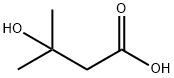
625-08-1
238 suppliers
$18.00/5g
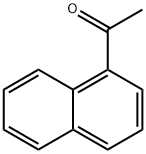
941-98-0
374 suppliers
$5.00/10g

75-25-2
341 suppliers
$16.00/50g
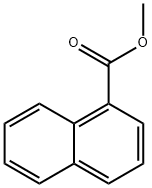
2459-24-7
102 suppliers
$33.00/5g
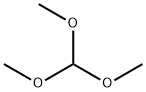
149-73-5
541 suppliers
$12.00/5g