Bimatoprost synthesis
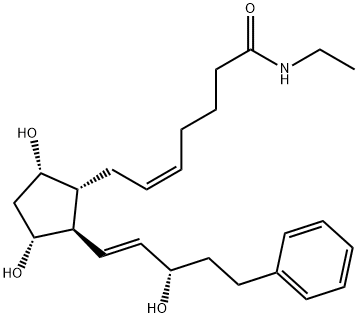
- Product Name:Bimatoprost
- CAS Number:155206-00-1
- Molecular formula:C25H37NO4
- Molecular Weight:415.57
1240483-19-5
2 suppliers
inquiry

155206-00-1
560 suppliers
$45.00/5mg
Yield:155206-00-1 91%
Reaction Conditions:
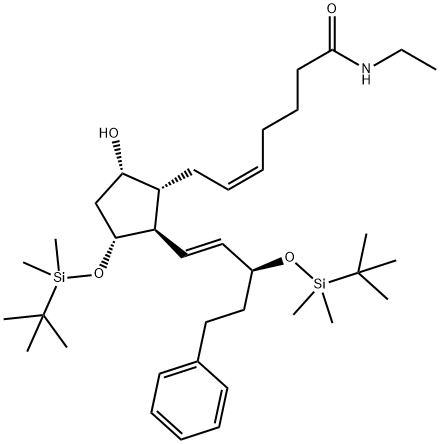
Stage #1: (Z)-7-((1R,2R,3R,5S)-3-(tert-butyldimethylsilyloxy)-2-((S,E)-3-(tert-butyldimethylsilyloxy)-5-phenylpent-1-enyl)-5-hydroxycyclopentyl)-N-ethylhept-5-enamidewith hydrogenchloride;water in tetrahydrofuran at 20; for 18 h;
Stage #2: in tetrahydrofuran; pH=6.8;Aqueous phosphate buffer;
Steps:
3
Preparation of the compound X (Bimatoprost)HCl 1.2 N (2 mL) is added at room temperature to a solution of the compound IX (950 mg, 1.5 mmoles) in a tetrahydrofuran/water 1:1 (50 mL) mixture, the reaction is performed under vigorous stirring and is complete after approximately 18 hours. It is quenched by adding phosphate buffer (pH = 6.8, 150 mL), then the organic phase is diluted with AcOEt, the two phases are separated, the aqueous phase is extracted with AcOEt, the re-combined organic phases are dried on magnesium sulphate and filtered, and lastly the solvent is removed at reduced pressure. The product is purified by means of column chromatography (AcOEt-methanol 95:5 v/v) . The Bimatoprost is obtained pure as a colourless oil with a yield of 91%.
References:
WO2010/97672,2010,A1 Location in patent:Page/Page column 20
856240-62-5
33 suppliers
inquiry
1201226-16-5
30 suppliers
inquiry

155206-00-1
560 suppliers
$45.00/5mg
![5-Heptenamide, N-ethyl-7-[(1R,2R,3R,5S)-5-hydroxy-2-[(1E,3S)-5-phenyl-3-[(tetrahydro-2H-pyran-2-yl)oxy]-1-penten-1-yl]-3-[(tetrahydro-2H-pyran-2-yl)oxy]cyclopentyl]-, (5Z)-](/CAS/20210111/GIF/856240-52-3.gif)
856240-52-3
1 suppliers
inquiry

155206-00-1
560 suppliers
$45.00/5mg
![5-Heptenoic acid, 7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]cyclopentyl]-, 3-hydroxy-2-(hydroxymethyl)-2-methylpropyl ester, (5Z)-](/CAS/20210111/GIF/1430731-70-6.gif)
1430731-70-6
1 suppliers
inquiry
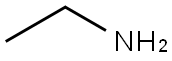
75-04-7
415 suppliers
$10.00/20mg

155206-00-1
560 suppliers
$45.00/5mg

75-04-7
415 suppliers
$10.00/20mg
![(Z)-7-[(1R,2R,3R,5S)-3,5-DIHYDROXY-2-((E)-(S)-3-HYDROXY-5-PHENYL-PENT-1-ENYL)-CYCLOPENTYL]-HEPT-5-ENOIC ACID METHYL ESTER](/CAS/GIF/38315-47-8.gif)
38315-47-8
66 suppliers
$64.00/1mg

155206-00-1
560 suppliers
$45.00/5mg