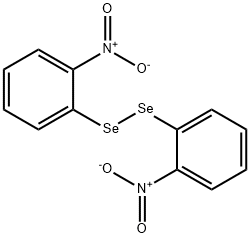
BIS(2-NITROPHENYL)DISELENIDE synthesis
- Product Name:BIS(2-NITROPHENYL)DISELENIDE
- CAS Number:35350-43-7
- Molecular formula:C12H8N2O4Se2
- Molecular Weight:402.12
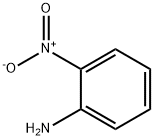
88-74-4
58 suppliers
$10.00/1g

35350-43-7
30 suppliers
inquiry
Yield:35350-43-7 88%
Reaction Conditions:
Stage #1: 2-nitro-anilinewith boron trifluoride diethyl etherate in dichloromethane at -15; for 0.25 h;
Stage #2: with n-Butyl nitrite in dichloromethane at -15 - 0; for 1 h;Further stages;
Steps:
4 2.1.4.Synthesisofbis(o-nitrophenyl)diselenide
The proceduresofCasaretal.werefollowed [33]. o-Nitroaniline(17mmol)wasdissolvedindrydichloromethane(25mL)at -15 °C. Borontrufluoride diethyletherate(3.5mL)wasaddeddropwiseandthemixturestirredfor15min.Asolutionofbutylnitrite (22.5mmol)indrydichloromethane(10mL)wasthenad-ded dropwiseandthemixturestirred30minat -15 °C, andanadditional 30minat0 °C. Coldpentane(20mL)wasaddedandthesolution filtered.Theprecipitatewaswashedwithcoldether(200mL).Thecollectedsolidwasthendissolvedinwater(80mL)on icebathandasolutionofKSeCN(17mmol)inwater(20mL)wasaddedover10min.AftercompleteadditionoftheKSeCNsolution, themixturewasstirredforanadditional10min.Theprecipitatewasthen filteredandcollected.Thesolidwasre-dis-solvedinethanol(60mL)andsodiummetal(17mmol)wasaddedportionwise.Themixturewasallowedtostirfor2hatwhichtimethe precipitatewas filtered,washedwithethanol(120mL),andallowedtodryovernighttogive bis(o-nitrophenyl)diselenide(o-NPdS, 3.0g,88%yield): 1H NMR(CDCl3) δ 8.39 (d,2H),7.92(d,2H),7.54-7.42(m,4H); 77Se-NMR (CDCl3, relativetomethylselenide) δ484.37, 77Se NMR(DMSO-d6, relativetomethylselenide) δ 478.76.
References:
Bianco, Christopher L.;Moore, Cathy D.;Fukuto, Jon M.;Toscano, John P. [Free Radical Biology and Medicine,2016,vol. 99,p. 71 - 78]
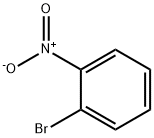
577-19-5
26 suppliers
$14.00/5g

35350-43-7
30 suppliers
inquiry
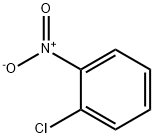
88-73-3
49 suppliers
$16.00/25g

35350-43-7
30 suppliers
inquiry
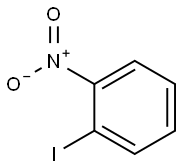
609-73-4
16 suppliers
$10.00/5g

35350-43-7
30 suppliers
inquiry
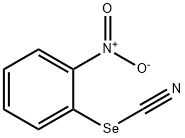
51694-22-5
73 suppliers
$44.00/1g

35350-43-7
30 suppliers
inquiry