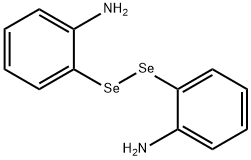
BIS(2-AMINOPHENYL)DISELENIDE synthesis
- Product Name:BIS(2-AMINOPHENYL)DISELENIDE
- CAS Number:63870-44-0
- Molecular formula:C12H12N2Se2
- Molecular Weight:342.16
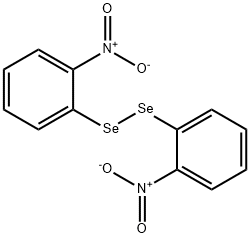
35350-43-7
30 suppliers
inquiry

63870-44-0
22 suppliers
inquiry
Yield: 84%
Reaction Conditions:
Stage #1:bis(o-nitrophenyl) diselenide with ferrous(II) sulfate heptahydrate in methanol;water for 1.5 h;Reflux;
Stage #2: with ammonium hydroxide in methanol;water for 0.166667 h;Reflux;
Steps:
General procedure for the synthesis of aniline-derived diselenides (3a-e)
General procedure: To the reduction of bis-o-nitrobenzene diselenides 2a-e (1.5 mmol), these was mixtured with methanol (25.0 mL), FeSO4.7H2O (6.5 mmol, 1.8 g) and H2O (25.0 mL) into a two-necked round-bottomed flask (100 mL) equipped with a reflux condenser. The reaction mixture was refluxed for 1.5 h, and then NH4OH (15.0 mL) was added and stirred for more 10 min under reflux. After this time, the solution was cooled to room temperature, diluted with ethyl acetate (60.0 mL) and washed with H2O (3 × 25.0 mL). The organic phase was separated, dried over Na2SO4 and concentrated under vacuum. The residue was purified by flash chromatography on silica gel using ethyl acetate/hexane as the eluent, obtaining the desired bis-aniline-derived diselenides 3a-e in good yields.
References:
Barbosa, Flavio A. R.;Bettanin, Luana;Botteselle, Giancarlo V.;Braga, Antonio L.;Canto, Rômulo F. S.;Ciancaleoni, Gianluca;Domingos, Josiel B.;Elias, Welman C.;Gallardo, Hugo;Rafique, Jamal;Saba, Sumbal;Salin, Drielly N. O. [Molecules,2021,vol. 26,# 15] Location in patent:supporting information
615-43-0
428 suppliers
$5.00/5g

63870-44-0
22 suppliers
inquiry

78163-72-1
0 suppliers
inquiry

63870-44-0
22 suppliers
inquiry
615-36-1
416 suppliers
$10.00/5g

63870-44-0
22 suppliers
inquiry
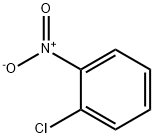
88-73-3
49 suppliers
$16.00/25g

63870-44-0
22 suppliers
inquiry