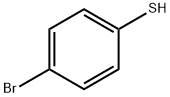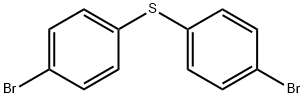
bis(4-bromophenyl) sulphide synthesis
- Product Name:bis(4-bromophenyl) sulphide
- CAS Number:3393-78-0
- Molecular formula:C12H8Br2S
- Molecular Weight:344.06
Yield:3393-78-0 97%
Reaction Conditions:
with Fe3O4SiO2-(Imine-Thiazole)-Cu(OAc)2 nanocomposite;PEG in N,N-dimethyl-formamide at 20; for 12 h;Reflux;
Steps:
Typical procedure for the domino synthesis of benzothiophene derivatives catalyzed by Fe3O4SiO2-(Imine-Thiazole)-Cu(OAc)2 nanocomposite
General procedure: A mixture of aryl halide (1 mmol), aromatic thiol (1 mmol), (1.0 equiv.) and Fe3O4SiO2-(Imine-Thiazole)-Cu(OAc)2 catalyst (25 mg) in PEG (2 mL) was stirred at 120 oC for 12 h. The reaction progress was followed by TLC (eluent: ether/ethyl acetate, 5:1). At the end of the reaction, the mixture was cooled to room temperature, ethyl acetate (10 mL) was added and the catalyst was separated by an eternal magnet. The organic phase was washed with water (2×10 mL) and dried over anhydrous MgSO4. The solvent was evaporated and the resulting crude material was purified by recrystallization from ether and ethyl acetate (5:1) to aord the pure product. The solid products was filtered and washed several times with petroleum ether and recrystallized using ethanol affords the desired products. All yields are reported after column chromatography. The products were identified and cited with reported literature. The purity of the products were confirmed by 1HNMR and 13CNMR spectroscopy.
References:
Feng, Meili;Yao, Wenjun;An, Jingjing;Yao, Yuze [Synthetic Communications,2022,vol. 52,# 22,p. 2122 - 2137] Location in patent:supporting information
139-66-2
304 suppliers
$5.00/10g

3393-78-0
70 suppliers
$22.00/1g
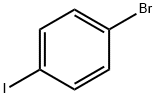
589-87-7
545 suppliers
$10.00/1g

3393-78-0
70 suppliers
$22.00/1g
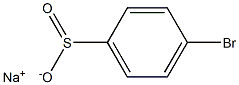
34176-08-4
27 suppliers
inquiry

3393-78-0
70 suppliers
$22.00/1g