
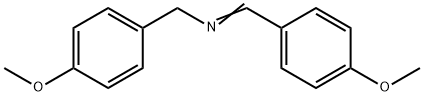
BIS-(4-METHOXY-BENZYL)-AMINE synthesis
- Product Name:BIS-(4-METHOXY-BENZYL)-AMINE
- CAS Number:17061-62-0
- Molecular formula:C16H19NO2
- Molecular Weight:257.33
3261-60-7
26 suppliers
inquiry

17061-62-0
177 suppliers
$6.00/1g
Yield:17061-62-0 100%
Reaction Conditions:
with sodium tetrahydridoborate in methanol at 5; for 2.75 h;Heating / reflux;
Steps:
5
p-Anisaldehyde (25.0 g, 0.1836 mol), 4-methoxybenzylamine (25.3 g, 0.1836 mol) and toluene (150 mL) were combined in a 500 mL round bottom flask which was fitted with a condenser and Dean-Stark trap under a N2 atmosphere. The solution was refluxed for 3 hours during which time 3 mL of H2O was azeotroped away from the reaction mixture. The reaction was cooled and concentrated on a rotovap at 400C for 2 hours. The clear, yellow oil was taken up in MeOH (150 mL) in a 500 mL round bottom flask fitted with a condenser under a N2 atmosphere. The reaction was cooled to 5°C, and NaBH4 was added in small portions over 45 min (off-gassing occurred). The reaction was slowly heated to reflux with vigorous off-gassing. After 2 hours at reflux, the reaction was cooled to room temperature and concentrated on the rotorvap at 300C for 2 hours, and then placed under high vacuum at 3O0C for 1 hour to give the title compound as a white crystalline solid (47.13 g, quantitative yield; 98.6 % purity by HPLC). MH+ = 258.1
References:
WO2007/109810,2007,A2 Location in patent:Page/Page column 112
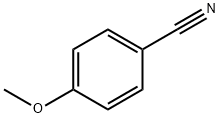
874-90-8
334 suppliers
$8.00/10g

17061-62-0
177 suppliers
$6.00/1g
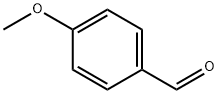
123-11-5
884 suppliers
$10.00/10g
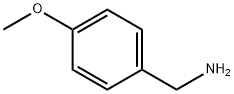
2393-23-9
474 suppliers
$20.00/25g

17061-62-0
177 suppliers
$6.00/1g

2393-23-9
474 suppliers
$20.00/25g

17061-62-0
177 suppliers
$6.00/1g