
bis(cyclohexyl) diselenide synthesis
- Product Name:bis(cyclohexyl) diselenide
- CAS Number:56592-97-3
- Molecular formula:C12H22Se2
- Molecular Weight:324.2231
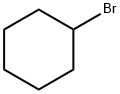
108-85-0
341 suppliers
$5.00/10g

56592-97-3
0 suppliers
inquiry
Yield:56592-97-3 87%
Reaction Conditions:
with selenium;hydrazine hydrate;sodium hydroxide in N,N-dimethyl-formamide at 25; for 8 h;Inert atmosphere;
Steps:
4.2. General procedure for the synthesis of diorganyl diselenides 1
General procedure: To a stirred mixture of Se (79 mg, 1.0 mmol, 1.0 eq) and NaOH (40 mg, 1.0 mmol, 1.0 eq) in DMF (1.5 mL) was added NH2NH2*H2O (36 mL, 0.75 mmol, 0.75 eq) under N2. The resulting mixture was stirred for 2 h at 25 °C during which it turned a reddish-brown. The alkyl halide was then added, and stirring was continued for 2 - 40 h at 25 - 153 °C until reaction completion (Table 2). The reaction mixture was then diluted with water (30 mL), extracted with CH2Cl2 (3 x 30 mL), and washed with brine (30 mL). Combined organic layers were dried (MgSO4) and concentrated in vacuo, forming a sticky residue that was purified by column chromatography (1:50/1:1 EtOAc/hexanes) to yield diorganyl diselenides 1. Spectral data of all compounds were in accordance with the literature information.
References:
Lim, Yoo Jin;Shin, Na Hye;Kim, Chorong;Kim, Ye Eun;Cho, Hyunsung;Park, Myung-Sook;Lee, Sang Hyup [Tetrahedron,2020,vol. 76,# 52,art. no. 131720]
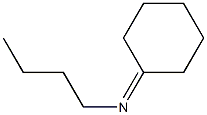
6407-39-2
0 suppliers
inquiry

56592-97-3
0 suppliers
inquiry
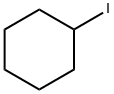
626-62-0
145 suppliers
$10.00/1g

56592-97-3
0 suppliers
inquiry
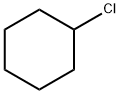
542-18-7
380 suppliers
$13.00/25g

56592-97-3
0 suppliers
inquiry
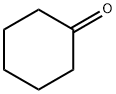
108-94-1
568 suppliers
$12.00/50g

56592-97-3
0 suppliers
inquiry