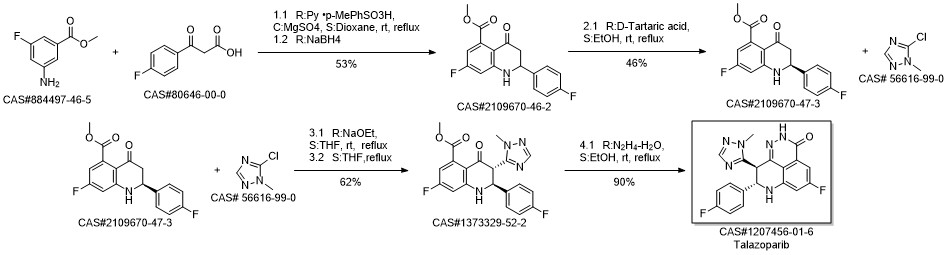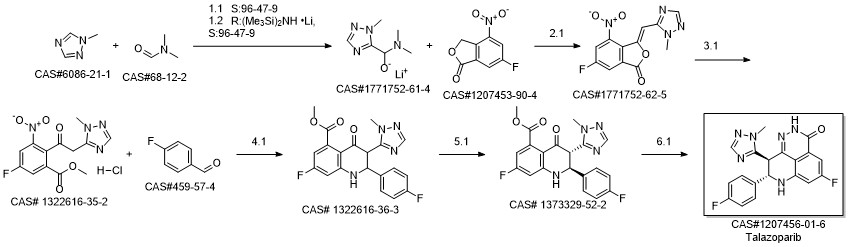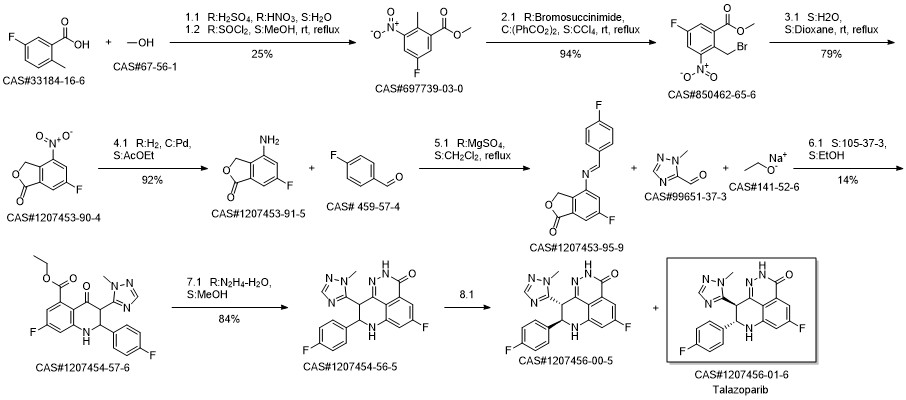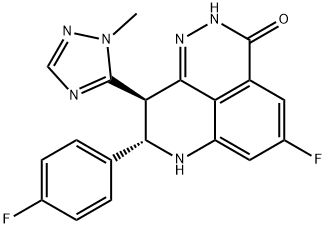
BMN 673 synthesis
- Product Name:BMN 673
- CAS Number:1207456-01-6
- Molecular formula:C19H14F2N6O
- Molecular Weight:380.35
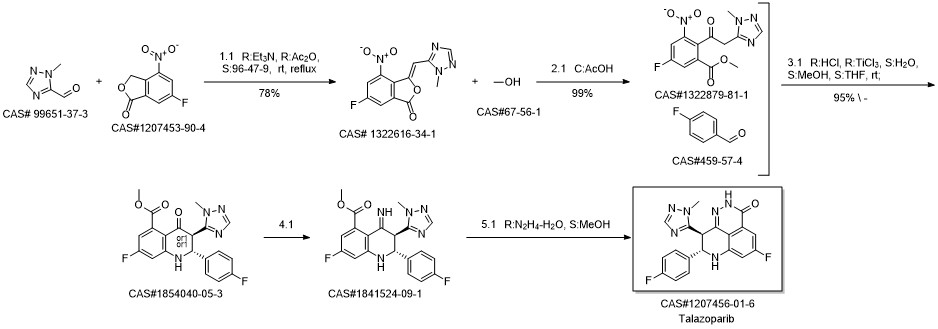
Reference: Wang, Bing; Chu, Daniel; Feng, Ying; Shen, Yuqiao; Aoyagi-Scharber, Mika; Post, Leonard E. Journal of Medicinal Chemistry. Discovery and Characterization of (8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-tetrahydro-3H-pyrido[4,3,2-de]phthalazin-3-one (BMN 673, Talazoparib), a Novel, Highly Potent, and Orally Efficacious Poly(ADP-ribose) Polymerase-1/2 Inhibitor, as an Anticancer Agent. Journal of Medicinal Chemistry. Volume 59. Issue 1. Pages 335-357. Journal; Online Computer File. (2016).
1207454-56-5
30 suppliers
$2970.00/500mg

1207456-01-6
180 suppliers
$28.00/1unit
1207456-00-5
55 suppliers
$109.00/5mg
Yield:1207456-00-5 90% ,1207456-01-6 78%
Reaction Conditions:
with CHIRALPAK IA in methanol;diethylamineResolution of racemate;
Steps:
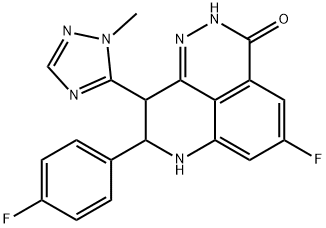
15 Example 15 (8R,95 -5-fluoro-8-(4-f1uorophenyl)-9-( l -methyl-lH-l ,2,4-triazoI-5-yl)-8,9-dihydro-2H-pyrido[4,3,2- ife]phthalazin-3(7No.)-one ( 1 a) and (8S,9R)-5-fluoro-8-(4-fiuoropheny l)-9-( 1 -methyl- 1 H- 1 ,2,4-triazol-5- ( 1 ) ( la) ( l b)
Example 15 (8R,95 -5-fluoro-8-(4-f1uorophenyl)-9-( l -methyl-lH-l ,2,4-triazoI-5-yl)-8,9-dihydro-2H-pyrido[4,3,2- ife]phthalazin-3(7No.)-one ( 1 a) and (8S,9R)-5-fluoro-8-(4-fiuoropheny l)-9-( 1 -methyl- 1 H- 1 ,2,4-triazol-5- ( 1 ) ( la) ( l b) 100394] A chiral resolution of 5-fluoro-8-(4-fluorophenyl)-9-( l-methyl- l - l ,2,4-triazol-5-yl)-8,9- dihydro-2H-pyrido[4,3,2-ife]phthalazin-3(7//)-one (1) (52.5 g) was carried out on a super-fluid chromatography (SFC) unit using a CHIRALPAK 1A column and C(¾/ methano l/di ethy lam ine (80/30/0.1 ) as a mobi le phase. This afforded two enantiomers with retention times of 7.9 minute (23.6 g. recovery 90 %, > 98 % ee) and 9.5 minute (20.4 g, recovery 78 %, > 98 % ee) as analyzed with a CHIRALPAK IA 0.46 cm x 15 cm column and C02/methanol/diethylamtne (80/30/0. 1 ) as a mobile phase at a flow rate of 2 g/minute.
References:
BIOMARIN PHARMACEUTICAL INC.;FENG, Ying;GUTIERREZ, Andres, A.;SHEN, Yuqiao;WANG, Evelyn, W.;OKHAMAFE, Augustus, O.;PRICE, Christopher, P.;CHOU, Tianwei WO2013/28495, 2013, A1 Location in patent:Paragraph 00394
1373329-52-2
12 suppliers
inquiry

1207456-01-6
180 suppliers
$28.00/1unit
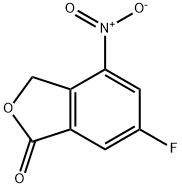
1207453-90-4
102 suppliers
$30.00/1g

1207456-01-6
180 suppliers
$28.00/1unit

1207456-00-5
55 suppliers
$109.00/5mg
![1(3H)-Isobenzofuranone,6-fluoro-3-[(1-Methyl-1H-1,2,4-triazol-5-yl)Methylene]-4-nitro-,(3Z)-](/CAS/20150408/GIF/1322616-34-1.gif)
1322616-34-1
15 suppliers
inquiry

1207456-01-6
180 suppliers
$28.00/1unit

1207456-00-5
55 suppliers
$109.00/5mg
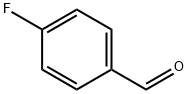
459-57-4
607 suppliers
$6.00/25g

1207456-01-6
180 suppliers
$28.00/1unit