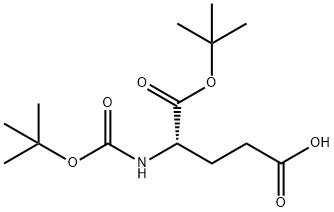
Boc-L-glutamic acid 1-tert-butyl ester synthesis
- Product Name:Boc-L-glutamic acid 1-tert-butyl ester
- CAS Number:24277-39-2
- Molecular formula:C14H25NO6
- Molecular Weight:303.35
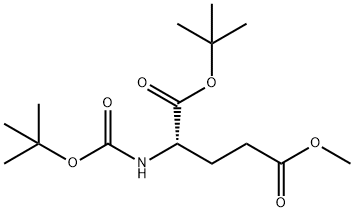
24277-38-1

24277-39-2
General procedure for the synthesis of Boc-L-glutamic acid 1-tert-butyl ester from (S)-1-tert-butyl 5-methyl 2-((tert-butoxycarbonyl)amino)pentanedioate: crude N-Boc-glutamic acid 1-tert-butyl ester (5-methyl ester from the previous step, 30.4 g, 95.8 mmol) was dissolved in THF (300 mL), 1N LiOH was added aqueous solution (144 mL). The reaction mixture was stirred at room temperature for 40 min and the progress of the reaction was monitored by TLC to confirm complete consumption of the feedstock. Upon completion of the reaction, THF was removed using a rotary evaporator.The remaining aqueous phase was extracted with ethyl acetate (3 × 100 mL) and subsequently acidified with solid citric acid to pH 4. The acidified aqueous phase was again extracted with ethyl acetate (3 × 100 mL). All the ethyl acetate extracts were combined, washed with saturated brine (2×50 mL), dried over anhydrous magnesium sulfate, filtered and concentrated to give the target product Boc-L-glutamic acid-1-tert-butyl ester as a colorless oil. Yield: 25 g (92% yield, calculated based on Boc-Glu(OMe)-OH). The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3) and 13C NMR (75 MHz, CDCl3).

24424-99-5
869 suppliers
$13.50/25G
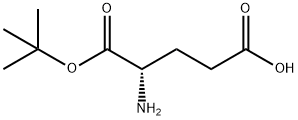
45120-30-7
290 suppliers
$5.00/1g

24277-39-2
284 suppliers
$6.00/1g
Yield:24277-39-2 73%
Reaction Conditions:
with triethylamine in 1,4-dioxane;water at 25; for 16 h;
Steps:
3 The synthesis of intermediates I-86 and I-87:
To a mixture of (4S)-4-amino-5-tert-butoxy-5-oxo-pentanoic acid (50 g, 246 mmol) in dioxane (150 mL) and H2O (150 mL) were added Et3N (49.7 g, 492 mmol) and Boc2O (59.1 g, 270 mmol). The mixture was stirred at 25°C for 16 h. The mixture was diluted with NaOH solution (2 M, 800 mL) and EtOAc (200 mL). The resulting solution was extracted with NaOH solution (2 M, 200 mL). HCl (aq., 6M) was added to the resulting aqueous layer above to adjust Ph~5. The resultant solution was extracted with EtOAc (500 mL × 2). The combined organic layers were washed with brine (300 mL), dried over anhydrous Na2SO4 and concentrated in vacuum to afford (S)-5-(tert-butoxy)-4-((tert-butoxycarbonyl)amino)-5- oxopentanoic acid (I-80) (55 g, 73% yield) as an orange oil.1H NMR (400 MHz, CDCl3) ^ 5.16 (d, J = 7.3 Hz, 1H), 4.29-4.15 (m, J = 5.0 Hz, 1H), 2.53-2.33 (m, 2H), 2.21-2.11 (m, 1H), 1.99-1.84 (m, 1H), 1.50-1.39 (m, 18H).
References:
WO2020/33784,2020,A1 Location in patent:Paragraph 00321

24277-38-1
41 suppliers
$420.00/25mg

24277-39-2
284 suppliers
$6.00/1g
![L-Glutamic acid, N-[(1,1-dimethylethoxy)carbonyl]-, 1-(1,1-dimethylethyl) 5-(phenylmethyl) ester](/CAS/20210305/GIF/28812-54-6.gif)
28812-54-6
0 suppliers
inquiry

24277-39-2
284 suppliers
$6.00/1g
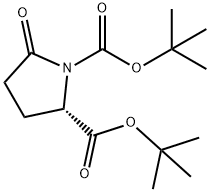
91229-91-3
126 suppliers
$7.00/250mg

24277-39-2
284 suppliers
$6.00/1g
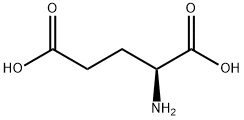
56-86-0
872 suppliers
$5.00/25g

24277-39-2
284 suppliers
$6.00/1g