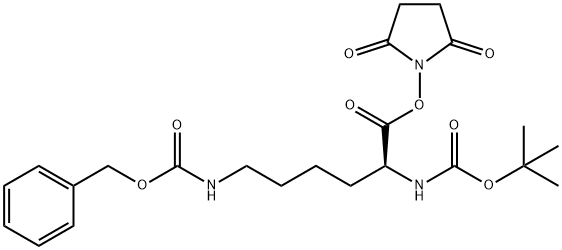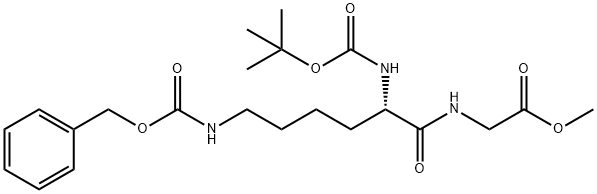
BOC-LYS(Z)-GLY-OME synthesis
- Product Name:BOC-LYS(Z)-GLY-OME
- CAS Number:23234-35-7
- Molecular formula:C22H33N3O7
- Molecular Weight:451.51
Yield:23234-35-7 98%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 20; for 16 h;
Steps:
36.i
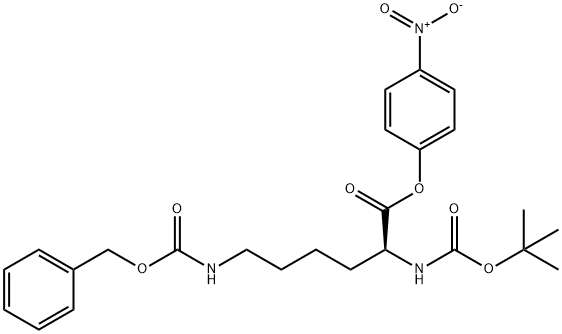
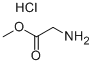
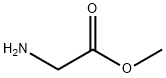
PNPO-α-Boc-ε-CBz-Lys (50.15 g, 0.100 mol) was added to a stirred suspension of methyl glycinate hydrochloride (12.56 g, 0.110 mol) in a mixture of triethylamine (30.36 g, 0.300 mol) and dimethylformamide (200 ml). After stirring at ambient temperature for 16 h, the volatile components were evaporated in vacuo and the residue partitioned between ethyl acetate (200 ml) and 5% aqueous sodium carbonate (175 ml). The aqueous phase was discarded and the ethyl acetate phase washed with more 5% aqueous sodium carbonate (4x200 ml) followed by 0.25 M hydrochloric acid (2x50 ml) and then with saturated aqueous sodium chloride (50 ml). The ethyl acetate solution was dried (magnesium sulphate), filtered and the solvent evaporated in vacuo to give MeOGIyLys [α-Boc] [ε-CBz] (44.39 g, 98%) as a colourless oil.1H nmr (300 MHz, CD3OD) δ (ppm): 1.3-1.8 (m, 6H); 1.44 (s, 9H); 3.13 (t, J 6.6 Hz, 2H); 3.70 (S1 3H); 3.88 (d, J 17.7 Hz, 1 H); 3.99 (d, J 17.7 Hz, 1 H); 4.04 (m, 1 H); 5.06 (s, 2H); 7.2-7.4 (m, 5H).LC/MS (Hydrophobic/Formate): ESI (+ve) observed [M + H]+ m/z = 452.0; calculated for C22H34N3O7 452.2. Rf (min) = 5.2.
References:
WO2007/82331,2007,A1 Location in patent:Page/Page column 110; 22/28
2389-45-9
317 suppliers
$6.00/1g

23234-35-7
12 suppliers
inquiry