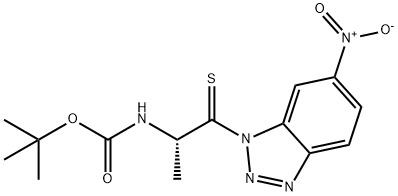
BOC-THIONOALA-1-(6-NITRO)BENZOTRIAZOLIDE synthesis
- Product Name:BOC-THIONOALA-1-(6-NITRO)BENZOTRIAZOLIDE
- CAS Number:184951-86-8
- Molecular formula:C14H17N5O4S
- Molecular Weight:351.38
![Carbamic acid, N-[(1S)-2-[(2-amino-5-nitrophenyl)amino]-1-methyl-2-thioxoethyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/184951-79-9.gif)
184951-79-9
0 suppliers
inquiry

184951-86-8
21 suppliers
inquiry
Yield:184951-86-8 90%
Reaction Conditions:
with acetic acid;sodium nitrite in water at 4; for 0.5 h;
Steps:
(S)-tert-Butyl (1-(6-nitro-1H-benzo[d][1,2,3]triazol-1-yl)-1-thioxopropan-2-yl)carbamate (A)
To a solution of (S)-tert-butyl (1-((2-amino-5-nitrophenyl)amino)-1-thioxopropan-2-yl)carbamate (0.300 mg, 0.88 mmol) in a mixture of acetic acid:water (95:5) at 4C was added sodium nitrite (97 mg, 1.41 mmol, 1.5 equiv.). After stirring the mixture for 30 min at 4C, chilled water was added and the precipitate then formed was filtered and dried to yield the final compound as a pale yellow solid (0.279 mg, 0.79 mmol, 90%),6 which spectral data matched the one published before.ADDIN CSL_CITATION { "citationItems" : [ { "id" : "ITEM-1", "itemData" : { "DOI" : "10.1021/jo961245q", "ISSN" : "00223263", "PMID" : "11667896", "author" : [ { "dropping-particle" : "", "family" : "Ashraf Shalaby", "given" : "M.", "non-dropping-particle" : "", "parse-names" : false, "suffix" : "" }, { "dropping-particle" : "", "family" : "Grote", "given" : "Christopher W.", "non-dropping-particle" : "", "parse-names" : false, "suffix" : "" }, { "dropping-particle" : "", "family" : "Rapoport", "given" : "Henry", "non-dropping-particle" : "", "parse-names" : false, "suffix" : "" } ], "container-title" : "Journal of Organic Chemistry", "id" : "ITEM-1", "issue" : "25", "issued" : { "date-parts" : [ [ "1996" ] ] }, "page" : "9045-9048", "title" : "Thiopeptide synthesis. u03b1-Amino thionoacid derivatives of nitrobenzotriazole as thioacylating agents", "type" : "article-journal", "volume" : "61" }, "uris" : [ "http://www.mendeley.com/documents/?uuid=b2f38ea2-934b-4306-9c29-f3341ca0f3c8" ] } ], "mendeley" : { "formattedCitation" : "5", "plainTextFormattedCitation" : "5", "previouslyFormattedCitation" : "5" }, "properties" : { "noteIndex" : 0 }, "schema" : "https://github.com/citation-style-language/schema/raw/master/csl-citation.json" }5 1H NMR (CDCl3): δ 9.61 (s, 1H), 8.46 (d, 1H, J = 9.1 Hz), 6.23-6.17 (m, 1H), 5.43 (d, 1H, J = 8.5 Hz), 1.64 (d, 3H, 6.9 Hz), 1.40 (s, 9H). HRMS (ESI) m/z: [M++1] calculated for C14H17N5O4S: 351.1001; observed: 351.1003.
References:
Soares, Pedro;Lucas, Xavier;Ciulli, Alessio [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 11,p. 2992 - 2995] Location in patent:supporting information
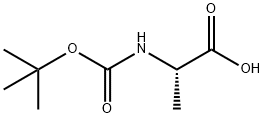
15761-38-3
528 suppliers
$5.00/10g

184951-86-8
21 suppliers
inquiry
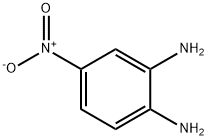
99-56-9
402 suppliers
$6.00/5g

184951-86-8
21 suppliers
inquiry
![Carbamic acid, N-[(1S)-2-[(2-amino-5-nitrophenyl)amino]-1-methyl-2-oxoethyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/184951-67-5.gif)
184951-67-5
0 suppliers
inquiry

184951-86-8
21 suppliers
inquiry