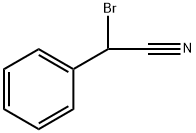
Bromobenzyl cyanide synthesis
- Product Name:Bromobenzyl cyanide
- CAS Number:5798-79-8
- Molecular formula:C8H6BrN
- Molecular Weight:196.05
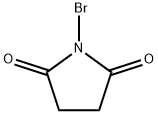
128-08-5
893 suppliers
$5.00/5 g
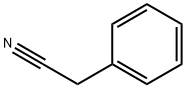
140-29-4
0 suppliers
$18.00/25mL

5798-79-8
50 suppliers
$55.00/50mg
Yield:-
Reaction Conditions:
with dibenzoyl peroxide for 5 h;Heating / reflux;
Steps:
2.1
Bromo (phenyl)acetonitrile To a solution of benzyl cyanide (4.93 mL, 42.7 mmol) in CC14 (275 mL) were added dibenzoyl peroxide (517 mg, 2.13 mmol) and N-bromosuccinimide (9.12 g, 51.2 mmol). The reaction mixture was heated to reflux and stirred for 5 hours and concentrated in vacuo to a volume of 100 mL. The solution was partitioned between CHCl3 and saturated NaHC03 solution, and the aqueous layer was extracted with several portions of CHC13. The combined organic layers were dried over Na2S04 and concentrated in vacuo to afford a yellow oil. Purification was achieved by flash column chromatography on silica gel using a gradient elution of 0-20% EtOAc/hexanes. Collection and concentration of the appropriate fractions yielded the title compound as a pale yellow oil. ¹H NMR (400 MHz, d6-DMSO) No. 7.61-7.58 (m, 2H), 7.53-7.45 (m, 3H), 6.59 (s, 1H)
References:
MERCK & CO., INC. WO2005/120516, 2005, A2 Location in patent:Page/Page column 47; 48

140-29-4
0 suppliers
$18.00/25mL

5798-79-8
50 suppliers
$55.00/50mg

140-29-4
0 suppliers
$18.00/25mL

5798-79-8
50 suppliers
$55.00/50mg
19472-74-3
254 suppliers
$5.00/5g
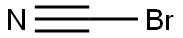
506-68-3
166 suppliers
$10.00/1g
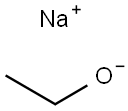
141-52-6
506 suppliers
$10.00/5g

140-29-4
0 suppliers
$18.00/25mL

5798-79-8
50 suppliers
$55.00/50mg
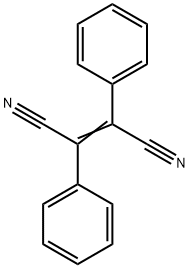
4591-16-6
4 suppliers
$144.00/50mg