
(Bromomethyl)cyclopropane synthesis
- Product Name:(Bromomethyl)cyclopropane
- CAS Number:7051-34-5
- Molecular formula:C4H7Br
- Molecular Weight:135
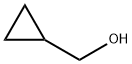
2516-33-8

7051-34-5
Example 1 (control experiment): 1250 mL of dimethylformamide (DMF) was added to the reaction vessel under nitrogen protection, followed by 280.8 g of triphenylphosphine. 70 g of hydroxymethylcyclopropane was added with stirring and the reaction was carried out at room temperature for 30 minutes. After cooling the reaction solution to -10°C, 158.3 g of bromine (102% of the theoretical amount) was slowly added dropwise over a period of 4 hours. Upon completion of the reaction, the reaction mixture was purified by distillation to afford bromomethylcyclopropane in 77.5% yield (based on theoretical values) with a product purity of >97%, which contained 0.6% open-chain haloalkanes.

2516-33-8
293 suppliers
$9.00/1g

7051-34-5
403 suppliers
$6.00/1g
Yield:7051-34-5 77.5%
Reaction Conditions:
with bromine;triphenylphosphine in N-methyl-acetamide
Steps:
1 Example 1
Example 1 (for comparison) 1250 ml of dimethylformamide were placed into the reaction vessel, and then 280.8 g of triphenylphosphine and then 70 g of hydroxymethylcyclopropane were added, the mixture was stirred for 30 minutes at room temperature under a nitrogen atmosphere, and the solution was subsequently cooled to -10° C. Then, 158.3 g of bromine (that is to say 2 mol % more than theoretically required) were metered in in the course of 4 hours. The reaction mixture was worked up by distillation. Bromomethylcyclopropane was obtained in a yield of 77.5% of theory. The purity of the product was over 97%, the open-chain halogenoalkanes amounted to 0.6%.
References:
Bayer Aktiengesellschaft US6008420, 1999, A

2516-33-8
293 suppliers
$9.00/1g
4399-47-7
232 suppliers
$14.14/1gm:

7051-34-5
403 suppliers
$6.00/1g

2516-33-8
293 suppliers
$9.00/1g

5162-44-7
366 suppliers
$10.00/5 g

4399-47-7
232 suppliers
$14.14/1gm:

7051-34-5
403 suppliers
$6.00/1g
756488-62-7
2 suppliers
inquiry

7051-34-5
403 suppliers
$6.00/1g

5911-08-0
205 suppliers
$25.00/1 g

5162-44-7
366 suppliers
$10.00/5 g

4399-47-7
232 suppliers
$14.14/1gm:

7051-34-5
403 suppliers
$6.00/1g