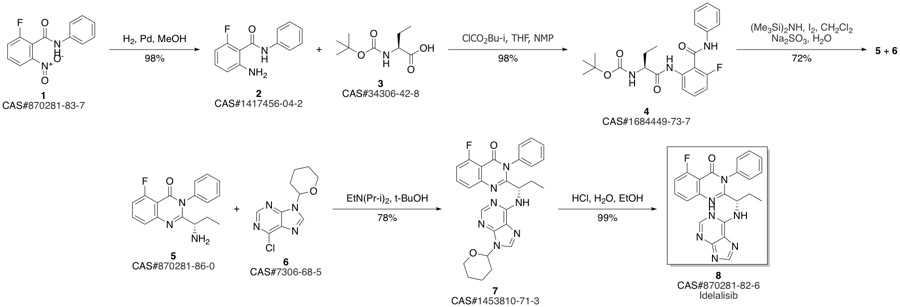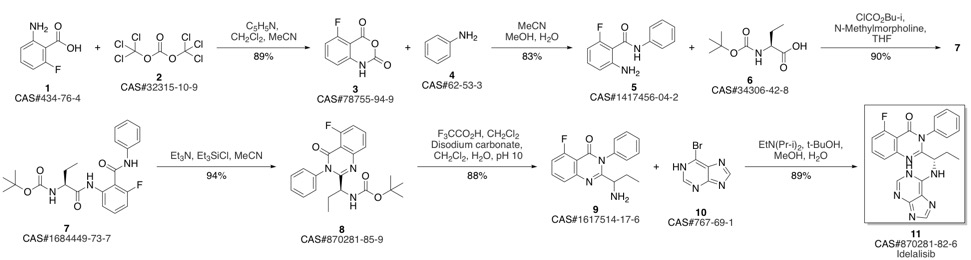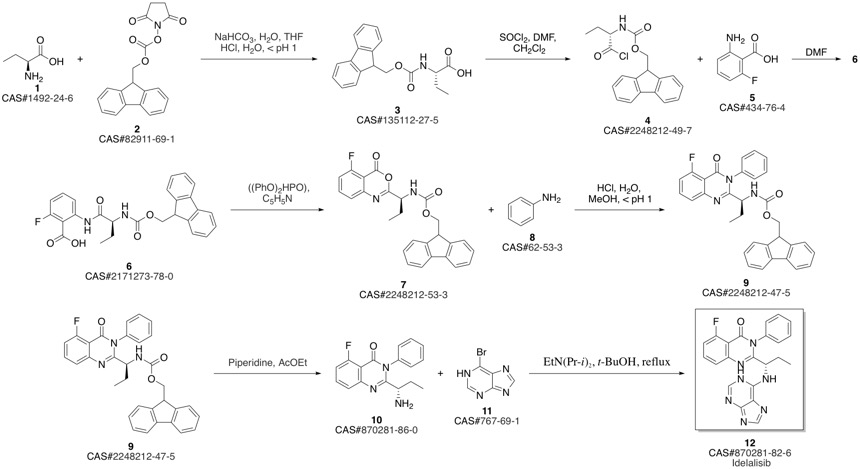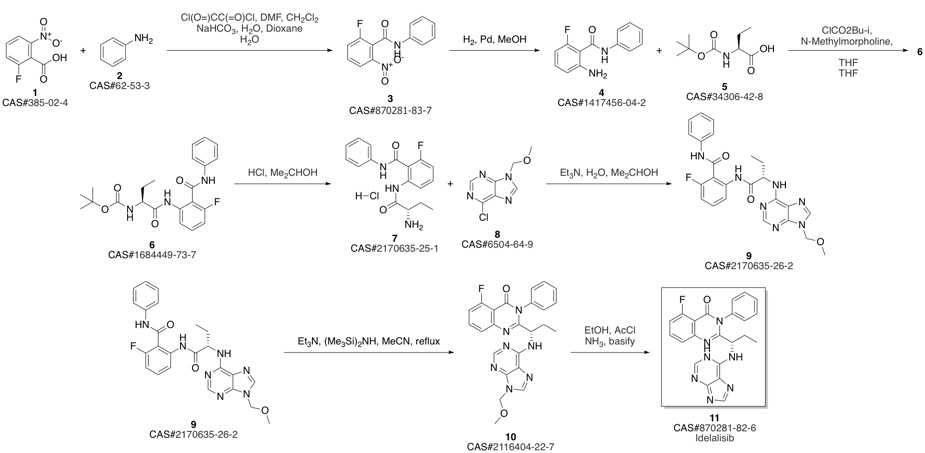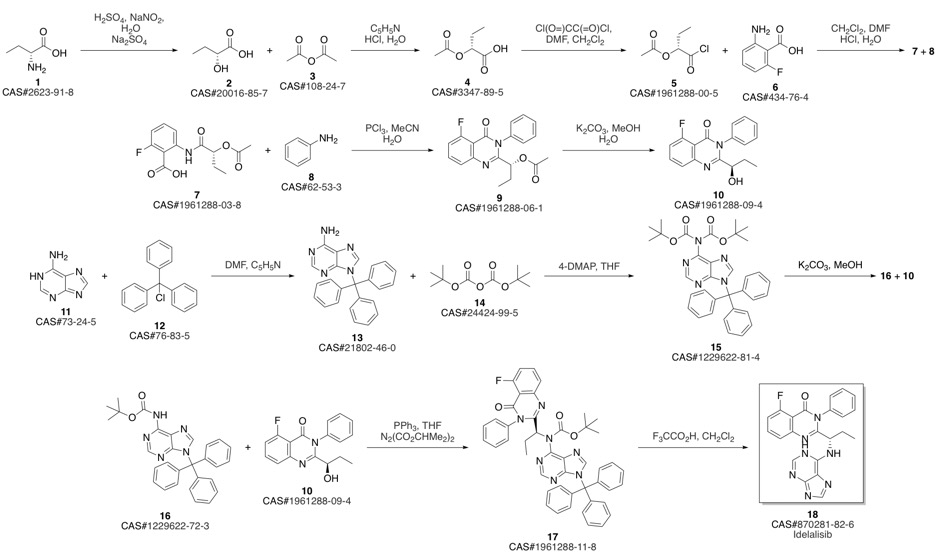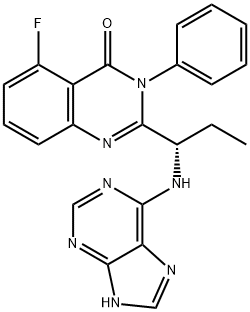
CAL-101 synthesis
- Product Name:CAL-101
- CAS Number:870281-82-6
- Molecular formula:C22H18FN7O
- Molecular Weight:415.42
Cai, Duo-Te; Zhang, Yue-Bin; Chen, Ken; Xiong, Qi-Xing; Luo, Wen-Juan; Gao, Zhi-Gang. Synthesis and evaluation of a novel heterocyclic compound against pediatric hepatoblastoma cells. Latin American Journal of Pharmacy. Volume 36. Issue 10. Pages 2022-2027. Journal. (2017).
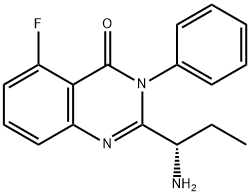
870281-86-0
124 suppliers
$23.00/100mg
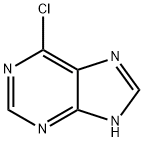
87-42-3
492 suppliers
$14.00/25g

870281-82-6
287 suppliers
$29.00/5mg
Yield:870281-82-6 3.1 g
Reaction Conditions:
with triethylamine in tert-butyl alcohol at 30 - 85; for 24 h;Concentration;Reagent/catalyst;
Steps:
28 Preparation of ldelalisib
(S)-2-(1 -aminopropyl)-5-fluoro-3-phenylquinazolin-4(3H)-one prepared in example 26 (4.2 g) and t-Butanol (21 mL) were charged into a 100 mL round bottom flask. Triethylamine (3.91 mL) and 6-Chloropurine (2.5 g) were added at 30 00. The resultant reaction mixture was heated to85°C and stirred for 24 hours. The reaction mixture was evaporated completely under reduced pressure at 40°C. The resultant residue was diluted with water (100 mL) and stirred for 30 minutes. The precipitate was filtered and the solid was washed with water (30 mL) and n-Hexane (50 mL) and dried for 1 hour under vacuum. The crude was purified by chromatographyusing Si02 (1 00:200) (solvent MeOH: DCM: TEA:: 5: 94: 1). The eluted fractions were evaporated completely under vacuum. The isolated product was diluted in dichloromethane (100 mL) and the organic layer was washed with brine solution (2x25 mL). The organic layer dried over sodium sulphate (10 g) and evaporated under reduced pressure to yield 3.1 g of Idelalisib as pale yellow solid.Purity: 97.87% by HPLC; chiral purity: 98.77% by H PLC
References:
WO2016/108206,2016,A2 Location in patent:Page/Page column 49; 50

62-53-3
686 suppliers
$10.00/1g

870281-82-6
287 suppliers
$29.00/5mg
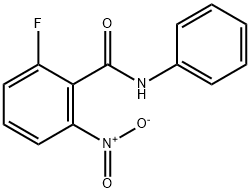
870281-83-7
78 suppliers
$8.00/250mg

870281-82-6
287 suppliers
$29.00/5mg
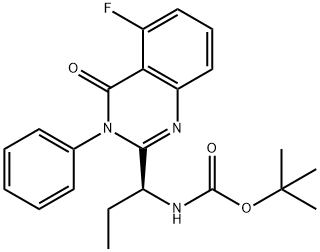
870281-85-9
94 suppliers
$29.00/1g

870281-82-6
287 suppliers
$29.00/5mg
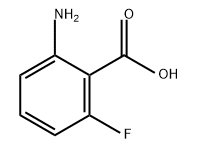
434-76-4
331 suppliers
$12.00/10g

870281-82-6
287 suppliers
$29.00/5mg