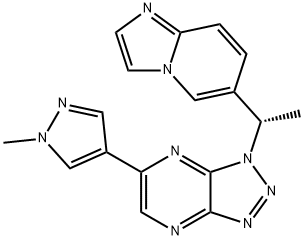
Savolitinib synthesis
- Product Name:Savolitinib
- CAS Number:1313725-88-0
- Molecular formula:C17H15N9
- Molecular Weight:345.36

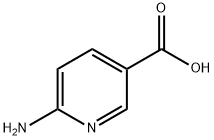
3167-49-5
399 suppliers
$8.00/5g

1313725-88-0
119 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 8 steps
1.1: water / 4 h / 75 - 80 °C / Large scale
2.1: 1,1'-carbonyldiimidazole / acetonitrile / 16 h / 10 - 20 °C / Large scale
2.2: 24 h / 5 - 12 °C / Large scale
3.1: tetrahydrofuran / 8 h / 5 - 20 °C / Large scale
4.1: isopropylamine hydrochloride; sodium tetraborate decahydrate; amine transaminase-436; pyridoxal 5'-phosphate; sodium hydroxide / water; dimethyl sulfoxide / 72 h / 44 - 54 °C / pH 10 / Enzymatic reaction
5.1: ammonia / water; dichloromethane / 10 - 20 °C
6.1: N-ethyl-N,N-diisopropylamine / 1-methyl-pyrrolidin-2-one / 120 °C
7.1: sodium nitrite; acetic acid / water / 4 h / 5 °C
7.2: 4 h / 25 °C
8.1: potassium carbonate / water; iso-butanol / 0.08 h / Inert atmosphere
8.2: 2 h / 30 - 65 °C / Inert atmosphere
References:
ASTRAZENECA AB;HUTCHISON MEDIPHARMA LIMITED;TURNER, Andrew, Roy;TURNER, Andrew, Timothy;HOWELL, Gareth, Paul;GALL, Malcolm, Allan, Young;MULHOLLAND, Keith, Raymond;ADLINGTON, Neil, Keith;TIAN, Zhenping;LIU, Bo;GONG, Qisun;YU, Wei WO2020/53198, 2020, A1
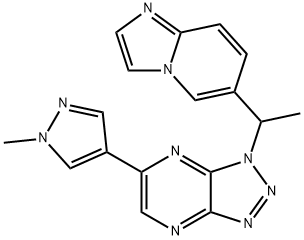
1313723-58-8
6 suppliers
inquiry
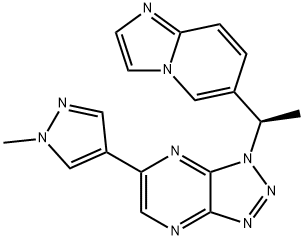
1313725-89-1
1 suppliers
inquiry

1313725-88-0
119 suppliers
inquiry
![N-methoxy-N-methylimidazo[1,2-a]pyridine-6-carboxamide](/CAS/20180629/GIF/1313726-20-3.gif)
1313726-20-3
3 suppliers
inquiry

1313725-88-0
119 suppliers
inquiry
![imidazo[1,2-a]pyridine-6-carboxylic acid](/CAS/GIF/139022-25-6.gif)
139022-25-6
162 suppliers
$10.00/250mg

1313725-88-0
119 suppliers
inquiry
![alpha-Methylimidazo[1,2-a]pyridine-6-methanamine](/CAS/20150408/GIF/1270475-03-0.gif)
1270475-03-0
33 suppliers
inquiry

1313725-89-1
1 suppliers
inquiry

1313725-88-0
119 suppliers
inquiry