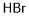Lithium bromide synthesis
- Product Name:Lithium bromide
- CAS Number:7550-35-8
- Molecular formula:BrLi
- Molecular Weight:86.85
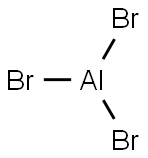
7727-15-3
181 suppliers
$17.46/1G
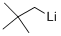
7412-67-1
8 suppliers
inquiry
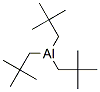
42916-36-9
2 suppliers
inquiry

7550-35-8
592 suppliers
$6.00/25g
Yield:42916-36-9 82.3%
Reaction Conditions:
in hexane;Addn. of neopentyllithium to a suspn. of AlBr3 in hexane at 0°C in 20 min under protective gas, refluxing (3 h).; Vac. distn. of hexane, sepn. of Al-compd. from LiBr by flask to flask vac. distn. at 120°C. Short-path vac. distn., elem. anal., mol wt.;
References:
Beachley Jr.;Victoriano [Organometallics,1988,vol. 7,# 1,p. 63 - 67]
17933-16-3
6 suppliers
inquiry
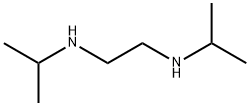
4013-94-9
161 suppliers
$35.00/5g
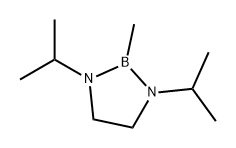
129920-20-3
0 suppliers
inquiry

7550-35-8
592 suppliers
$6.00/25g

17933-16-3
6 suppliers
inquiry
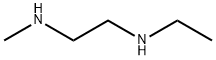
111-37-5
21 suppliers
$398.00/5g

129920-21-4
0 suppliers
inquiry

7550-35-8
592 suppliers
$6.00/25g