
CBI-BB ZERO/001711 synthesis
- Product Name:CBI-BB ZERO/001711
- CAS Number:35212-90-9
- Molecular formula:C10H8N2O4S
- Molecular Weight:252.25
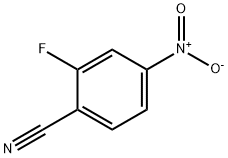
34667-88-4
125 suppliers
$10.00/1g
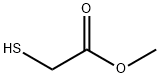
2365-48-2
334 suppliers
$14.00/25g

35212-90-9
12 suppliers
inquiry
Yield:35212-90-9 54%
Reaction Conditions:
with triethylamine in acetonitrile at 25; for 19 h;
Steps:
44 Methyl 3-amino-6-nitrobenzothiophene-2-carboxylate
Methyl 3-amino-6-nitrobenzothiophene-2-carboxylate: Methyl thioglycolate (0.08 mL, 0.85 mmol) was added to a solution of 2-fluoro-4-nitrobenzonitrile (145 mg, 0.87 mmol), and triethylamine (0.14 mL, 1.0 mmol) in acetonitrile (20 mL) stirred under nitrogen at 25° C. After 3 hours further triethylamine (0.28 mL, 2.0 mmol) was added to the solution, which was stirred at 25° C. for a further 16 hours. The solvent was removed under reduced pressure to give a brown residue, which upon trituration with chloroform precipitated methyl 3-amino-6-nitrobenzothiophene-2-carboxylate (103 mg, 54%) as a red brown solid, mp 228.5-229.5° C. 1H NMR (DMSO-d6): δ 8.87 (d, J=2.0 Hz, 1H), 8.32 (d, J=9.0 Hz, 1H), 8.15 (dd, J=8.8, 2.0 Hz, 1H), 7.26 (brs, 2H), 3.77 (s, 3H). Mass Spectrum (CI): 253 (100, MH+), 252 (52, M+).
References:
US6344459,2002,B1 Location in patent:Page column 71
4110-33-2
15 suppliers
$24.73/1gm:
584-08-7
1163 suppliers
$5.00/100g
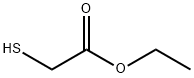
623-51-8
230 suppliers
$7.00/25g

35212-90-9
12 suppliers
inquiry
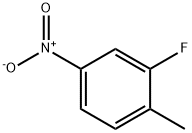
1427-07-2
340 suppliers
$6.00/5g

35212-90-9
12 suppliers
inquiry
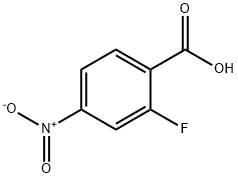
403-24-7
343 suppliers
$5.00/250mg

35212-90-9
12 suppliers
inquiry
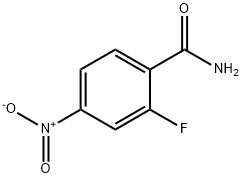
350-32-3
28 suppliers
$185.00/1g

35212-90-9
12 suppliers
inquiry