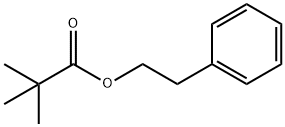
CENTIFOLYL synthesis
- Product Name:CENTIFOLYL
- CAS Number:67662-96-8
- Molecular formula:C13H18O2
- Molecular Weight:206.28
Yield: 82%
Reaction Conditions:
with iron(III)-acetylacetonate;sodium carbonate in n-heptane at 105; for 24 h;Inert atmosphere;
Steps:
5.4. Representative transesterification procedure catalyzed by Fe(acac)3 and Na2CO3 as additive
General procedure: To a 25 mL, one-necked, round-bottomed flask was placed Fe(acac)3 (36 mg, 0.10 mmol, 5 mol %), benzyl alcohol (216 mg, 208 μL, 2 mmol), and Na2CO3 (10.6 mg, 0.10 mmol, 5 mol %) in 10 mL heptane at room temperature under nitrogen atmosphere. A solution of methyl benzoate (272 mg, 256 μL, 2 mmol) in heptane (10 mL) was added via syringe. The resulting mixture was heated to reflux with the removal of the methanol by Dean-Stark apparatus and the reaction progress was monitored by TLC, 1H NMR spectroscopy, and GC analysis until completion of the reaction (6 h). The reaction mixture was then gradually cooled to room temperature and quenched with saturated aqueous NH4Cl solution (5 mL), then extracted with 20 mL ethyl acetate. The combined organic layer was dried (anhydrous MgSO4), filtered, and evaporated to give a crude product that was purified by column chromatography on silica gel (hexane/AcOEt=50/1) to provide the pure benzyl benzoate product 412 mg, 97% yield.
References:
Weng, Shiue-Shien;Ke, Chih-Shueh;Chen, Fong-Kuang;Lyu, You-Fu;Lin, Guan-Ying [Tetrahedron,2011,vol. 67,# 9,p. 1640 - 1648] Location in patent:experimental part
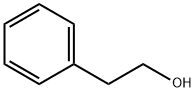
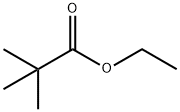
60-12-8
598 suppliers
$6.00/25g
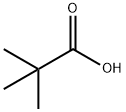
1538-75-6
226 suppliers
$17.67/10gm:

67662-96-8
32 suppliers
inquiry

60-12-8
598 suppliers
$6.00/25g
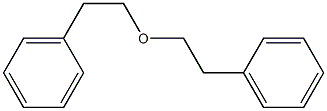
3282-30-2
380 suppliers
$18.00/100ml

67662-96-8
32 suppliers
inquiry