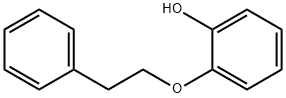
CHEMBRDG-BB 4003142 synthesis
- Product Name:CHEMBRDG-BB 4003142
- CAS Number:33130-24-4
- Molecular formula:C14H14O2
- Molecular Weight:214.26
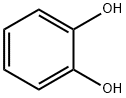
120-80-9
531 suppliers
$13.00/25g
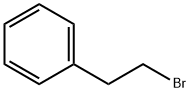
103-63-9
504 suppliers
$9.00/10g

33130-24-4
12 suppliers
$31.00/250mg
Yield:33130-24-4 19%
Reaction Conditions:
with potassium carbonate in acetonitrile at 80; for 18 h;
Steps:
2-Phenethoxyphenol (8) [CAS Reg. No. 33130-24-4]
A procedure for the preparation of alkyl aryl ethers was adopted.48Phenethyl bromide (1.853 g, 10 mmol), catechol (7b; 1.103 g, 10mmol), and K2CO3 (2.075 g, 15 mmol) were added to MeCN (40 mL),and the mixture was stirred at 80 °C for 18 h before quenching withaq 2 M HCl (20 mL). After extraction with EtOAc (3 × 50 mL), the organicphases were combined, washed with brine, and dried (anhydMgSO4). After filtration, the solvents were removed on a rotary evaporator,and the residue was purified by column chromatography (eluent:PE/EtOAc 10:1) to afford the product as a colorless liquid; yield:0.393 g (19%), which solidified after standing overnight at r.t.; mp 45-46 °C (Lit.41 mp 48 °C); Rf = 0.54 (PE/EtOAc 10:1).1H NMR (400 MHz, CDCl3): δ = 7.39-7.20 (m, 5 H), 6.92-6.81 (m, 4 H),5.50 (s, 1 H), 4.26 (t, J = 6.8 Hz, 2 H), 3.12 (t, J = 6.8 Hz, 2 H).13C NMR (100 MHz, CDCl3): δ = 145.94, 145.63, 137.87, 128.89,128.71, 126.76, 121.77, 120.12, 114.67, 112.16, 69.58, 35.78.
References:
Sang, Dayong;Tian, Juan;Tu, Xiaodong;He, Zhoujun;Yao, Ming [Synthesis,2019,vol. 51,# 3,p. 704 - 712]
161470-27-5
1 suppliers
inquiry

33130-24-4
12 suppliers
$31.00/250mg

100-39-0
430 suppliers
$10.00/10g

120-80-9
531 suppliers
$13.00/25g
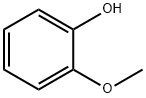
90-05-1
791 suppliers
$5.00/1g