CHEMBRDG-BB 4022614 synthesis
- Product Name:CHEMBRDG-BB 4022614
- CAS Number:23600-44-4
- Molecular formula:C14H14N2O
- Molecular Weight:226.27
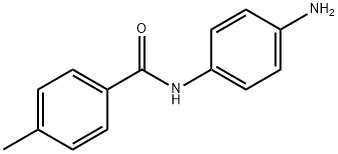
33667-88-8
5 suppliers
$28.60/50MG

23600-44-4
15 suppliers
$46.00/1g
Yield:23600-44-4 62%
Reaction Conditions:
with iron(III) chloride;pyrographite;hydrazine hydrate in ethanol at 85; for 2 h;
Steps:
1.2. General procedure for the preparation of the N-(4-aminophenyl)-2-methylbenzamide derivatives 13a-u
General procedure: A mixture of 12a-u (3.8 mmol) in ethanol (40 mL) was heated to 60 oC. FeCl3 (0.1 g) and activated charcoal (0.5 g) were added, and the mixture was heated to 85 oC. Hydrazine hydrate (8 mmol, 4 mL) was added dropwise, and the reaction was stirred for 2 h. Upon completion, the mixture was filtered, washed with ethanol, and concentrated in vacuo to afford compounds 13a-u.
References:
Wang, Ru;Liu, Hu;You, Yuan-Yuan;Wang, Xin-Yu;Lv, Bing-Bing;Cao, Li-Qin;Xue, Jia-Yu;Xu, Yun-Gen;Shi, Lei [Bioorganic and Medicinal Chemistry Letters,2021,vol. 36,art. no. 127788] Location in patent:supporting information
874-60-2
413 suppliers
$10.00/5g

23600-44-4
15 suppliers
$46.00/1g
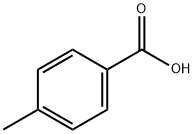
99-94-5
611 suppliers
$9.00/1g

23600-44-4
15 suppliers
$46.00/1g
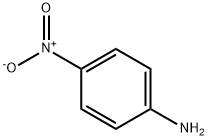
100-01-6
72 suppliers
$11.19/25G

23600-44-4
15 suppliers
$46.00/1g