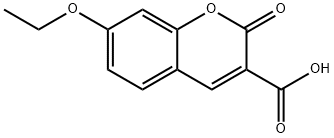
CHEMBRDG-BB 5867144 synthesis
- Product Name:CHEMBRDG-BB 5867144
- CAS Number:79065-74-0
- Molecular formula:C12H10O5
- Molecular Weight:234.2
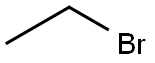
74-96-4
442 suppliers
$9.00/5g

105-53-3
792 suppliers
$5.00/25g
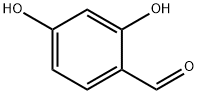
95-01-2
492 suppliers
$10.00/1g

79065-74-0
14 suppliers
$46.00/1g
Yield:79065-74-0 98.3%
Reaction Conditions:
Stage #1: diethyl malonate;2,4-Dihydroxybenzaldehydewith piperidine;acetic acid in ethanol; for 30 h;Reflux;
Stage #2: ethyl bromidewith potassium carbonate in N,N-dimethyl-formamide at 20; for 48 h;
Stage #3: with ethanol;sodium hydroxide for 0.25 h;Reflux;
Steps:
18
Add 2,4-dihydroxybenzaldehyde (2.76g, 20mmol), diethyl malonate (3.84g, 24mmol), piperidine (0.2mL), 2 drops of acetic acid, ethanol (24mL) into a 100mL single-necked flask, The reaction was refluxed for 30 hours. After the reaction was completed, it was cooled and poured into 40 mL of ice water to precipitate a solid, filtered, washed with 50% ice ethanol, and recrystallized with 50% ice ethanol to obtain a yellow solid (3.53 g) with a yield of 75.5%.The yellow solid (2.34 g, 10 mmol), potassium carbonate (1.66 g, 12 mmol), DMF (25 mL) were added to a 100 mL single-neck flask, and ethyl bromide (1.64 g, 15 mmol) was added dropwise, and reacted at room temperature for 48 hours.After the reaction, 30 mL of water was added to precipitate a solid, which was filtered and dried to obtain a white solid (2.44 g) with a yield of 93.2%.Add the above white solid (2.62g, 10mmol), 10% sodium hydroxide (15mL), ethanol (15mL) to a 100mL single-necked flask, and react under reflux for 15min. After the reaction is over, add hydrochloric acid dropwise to adjust pH 2, precipitate solids, and filter. After washing with water and drying, a white solid 3-carboxy-7-ethoxycoumarin (2.30g) was obtained with a yield of 98.3%.
References:
CN110804045,2020,A Location in patent:Paragraph 0258; 0262-0264
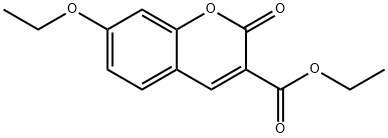
79065-62-6
1 suppliers
inquiry

79065-74-0
14 suppliers
$46.00/1g
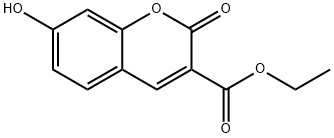
6093-71-6
109 suppliers
$28.00/5mg

79065-74-0
14 suppliers
$46.00/1g

95-01-2
492 suppliers
$10.00/1g

79065-74-0
14 suppliers
$46.00/1g