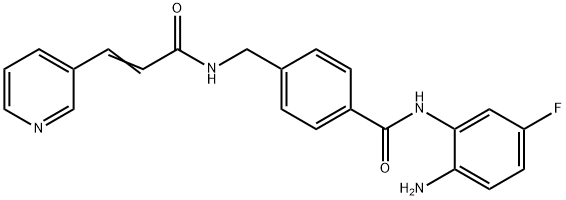
Chidamide synthesis
- Product Name:Chidamide
- CAS Number:743420-02-2
- Molecular formula:C22H19FN4O2
- Molecular Weight:390.41
367-31-7
218 suppliers
$8.00/1g
219964-34-8
14 suppliers
inquiry

743420-02-2
129 suppliers
$39.00/1mg
Yield:743420-02-2 57%
Reaction Conditions:
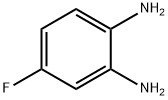
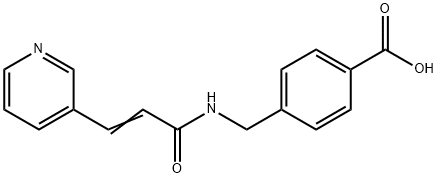
Stage #1:4-[N-(pyridin-3-ylacryloyl)aminomethyl]benzoic acid with 1,1'-carbonyldiimidazole in tetrahydrofuran at 45; for 1 h;
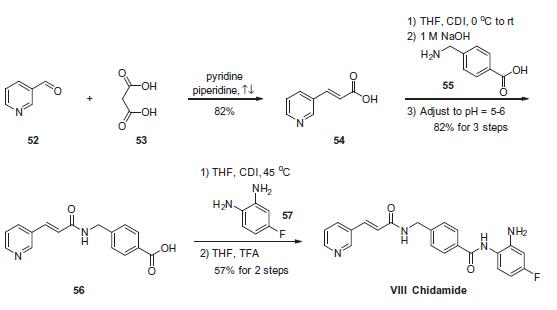
Stage #2:4-fluoro-1,2-phenylenediamine with trifluoroacetic acid in tetrahydrofuran at 20; for 24 h;
Steps:
2 Example 2
To a suspension of 0.29 g (1.78 mmol) of N, N’-carbonyldiimidazole in tetrahydrofuran (15 ml) is added 0.50 g (1.78mmol)) of 4-[N-Pyridin-3-ylacryloyl)aminomethyl]benzoic acid, followed by stirring at 45°C for 1 hour. After cooling, thereaction mixture is added to a separately prepared furan tetrahydrofuran (10 ml) solution including 0.28 g (2.22 mmol)of 4-fluoro-1,2-phenylenediamine and 0.20 g (1.78 mmol) of trifluoroacetic acid at room temperature. After reaction atroom temperature for 24 hours, the deposited white solid is collected by filtration, washed with tetrahydrofunan, andthen dried to give the title compound (0.40 g, 57%). 1H NMR (300 MHz, DMSO-d6): δppm: 4.49 (2H, d), 4.84 (2H, br.s),6.60 (1H, t), 6.80 (2H, m), 6.96 (1H, t), 7.18 (1H, d), 7.42 (2H, d), 7.52 (1H, d), 7.95 (2H, d), 8.02 (1H, d), 8.56 (1H, d),8.72 (1H, br. t), 8.78 (1H, s), 9.60 (1H, br.s). 1R (KBr) cm-1: 3310, 1655, 1631, 1524, 1305, 750. HRMS calcd forC22H19N4O2F: 390.4170. Found: 390.4172. MA calcd for C22H19O2F: C, 67.68%; H, 4.40%; N, 14.35. Found: C, 67,52%; H, 4.38%; N, 14.42%.
References:
Shenzhen Chipscreen Biosciences Ltd.;Lu, Xian-Ping;Li, Zhibin;Xie, Aihua;Li, Boyu;Ning, Zhiqiang;Shan, Song;Deng, Tuo;Hu, Weiming;Shi, Leming EP2860174, 2017, B1 Location in patent:Paragraph 0040; 0041