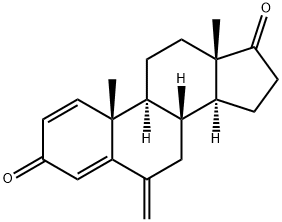
Exemestane synthesis
- Product Name:Exemestane
- CAS Number:107868-30-4
- Molecular formula:C20H24O2
- Molecular Weight:296.4
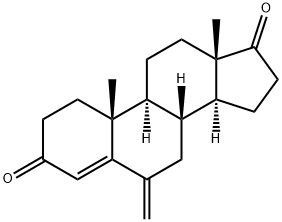
19457-55-7
131 suppliers
$46.00/1g

107868-30-4
528 suppliers
$28.00/5mg
Yield:107868-30-4 81.8%
Reaction Conditions:
with N,O-Bis(trimethylsilyl)trifluoroacetamide;chloranil;trifluorormethanesulfonic acid in toluene at 108 - 110; for 0.75 h;Product distribution / selectivity;Heating / reflux;
Steps:
1
EXAMPLE 1 Obtaining 6-methylenandrost-1,4-dien-3,17-dione; [Show Image] A mixture of 1 g of 6-methylenandrost-4-en-3,17-dione (3.5 mmol), 0.91 g of 2,3,5,6-tetrachloro-1,4-benzoquinone (chloranil) (3.7 mmol), 80 ml of toluene, 0.03 ml of trifluoromethanesulfonic acid (0.35 mmol) and 3.74 ml of bis(trimethylsilyl)trifluoroacetamide (BSTFA) (14.1 mmol) is stirred at reflux temperature (about 108-110°C) for 45 minutes. The mixture is then cooled at room temperature (20-22°C) and washed 6 times with a 2% aqueous sodium hydroxide solution (20 ml each time) and 3 times with a 30% aqueous sodium chloride solution. The organic phase is evaporated under reduced pressure, the solvent is substituted with heptane and the suspension is cooled. The product is filtered, washed with cold heptane and dried, obtaining 0.81 g [Yield : 81.8%] of crude 6-methylenandrost-1,4-dien-3,17-dione. The product can be purified by recrystallization in solvents or mixtures of solvents (ethyl acetate, ethyl acetate/heptane, ethanol or ethanol/water). The recrystallized solid has a melting point (m.p.) of 191-194°C and the following spectroscopic characteristics: 1H-NMR (DMSO-d6) : 0.83 (3H, s, CH3 18), 1.09 (3H, s, CH3 19), 1.30-1.80 (10 H), 1.99 (1H, dt, J 9.6; 18.8 Hz, H16), 2.39 (1H, dd, J 8.8; 18.8 Hz, H16), 5.01 (2H, s, H 6a), 5.96 (1H, s, H4), 6.12 (1H, d, J 10.0 Hz, H2), 7.22 (1H, d, J 10.0 Hz, H1). 13C-NMR (DMSO-d6): 14.1 (CH3 18), 20.1 (CH3 19), 22.0 (CH2), 22.2 (CH2), 31.5 (CH2), 35.3 (CH), 35.9 (CH2), 39.2 (CH2), 44.1 (C 13), 47.7 (C 10), 49.9 (CH), 50.3 (CH), 112.8 (C 6 a), 122.4 (C4), 127.6 (C3), 146.1 (C5), 155.8 (C1), 168.2 (C6), 185.7 (C3), 219.7 (C17).
References:
Crystal Pharma, S.A. EP2070943, 2009, A1 Location in patent:Page/Page column 9
933455-74-4
0 suppliers
inquiry

107868-30-4
528 suppliers
$28.00/5mg
861395-77-9
12 suppliers
inquiry

107868-30-4
528 suppliers
$28.00/5mg
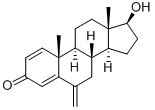
122370-91-6
68 suppliers
$215.00/1mg

107868-30-4
528 suppliers
$28.00/5mg
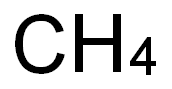
7440-44-0
352 suppliers
$12.00/25g

19457-55-7
131 suppliers
$46.00/1g
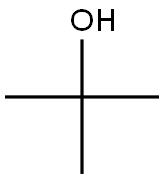
75-65-0
775 suppliers
$10.00/10ml

107868-30-4
528 suppliers
$28.00/5mg