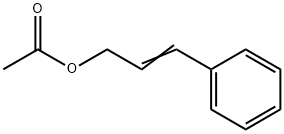
Cinnamyl acetate synthesis
- Product Name:Cinnamyl acetate
- CAS Number:103-54-8
- Molecular formula:C11H12O2
- Molecular Weight:176.21
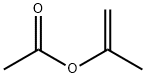
108-22-5
267 suppliers
$19.00/25mL
104-54-1
653 suppliers
$11.30/5g

103-54-8
392 suppliers
$9.00/25g
Yield:103-54-8 97%
Reaction Conditions:
with toluene-4-sulfonic acid in acetonitrile at 20; for 16 h;
Steps:
General procedure for the protection of alcohols 1 as acetyl-derivatives.
General procedure: A mixture of the alcohol 1 (1 mmol), isopropenyl acetate (0.45 mL, 4 mmol) and p-TsOH (4 mg, 0.02 mmol) in dichloromethane or acetonitrile (4 mL) was stirred the indicated temperature (see Tables 1 and 2). Reaction times ranged from 16 to 36 h. After completion of the reaction (TLC monitoring) the mixture was poured into 10 mL of 10% aqueous sodium hydrogen carbonate solution. The aqueous phase was extracted with ethyl acetate (3 × 10 mL) and the combined organic phases were washed with 10 mL of brine, dried over sodium sulfate and then evaporated under vacuum. Purification by silica gel column chromatography afforded the pure acetyl ester 2. Physical and spectral data of known compounds were consistent with the ones reported in literature. Physical and spectral data of not previously described compounds are reported below.
References:
Temperini, Andrea;Minuti, Lucio;Morini, Tommaso;Rosati, Ornelio;Piazzolla, Francesca [Tetrahedron Letters,2017,vol. 58,# 43,p. 4051 - 4053]

104-54-1
653 suppliers
$11.30/5g

141-78-6
683 suppliers
$20.50/250ml

103-54-8
392 suppliers
$9.00/25g
300-57-2
231 suppliers
$51.00/25mL

64-19-7
1628 suppliers
$10.00/25ML

103-54-8
392 suppliers
$9.00/25g
673-32-5
136 suppliers
$11.00/1g

64-19-7
1628 suppliers
$10.00/25ML

103-54-8
392 suppliers
$9.00/25g
