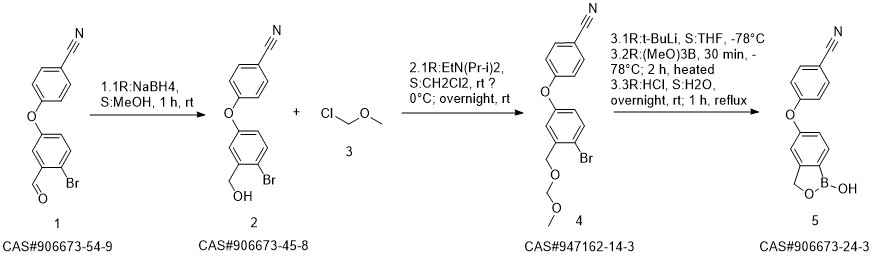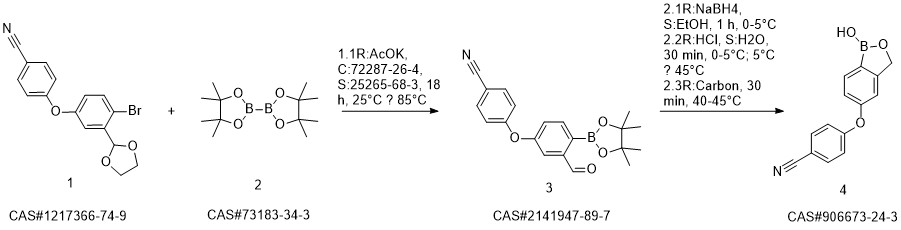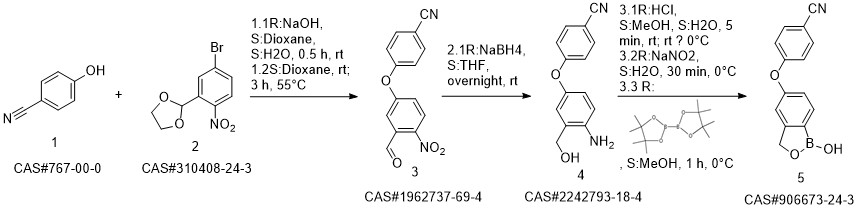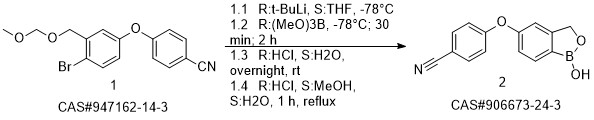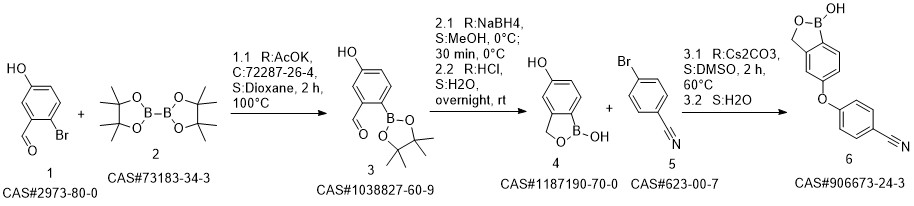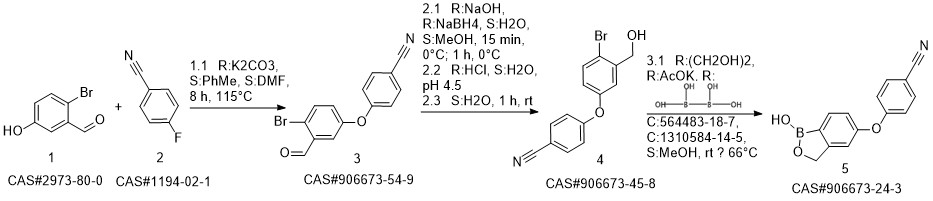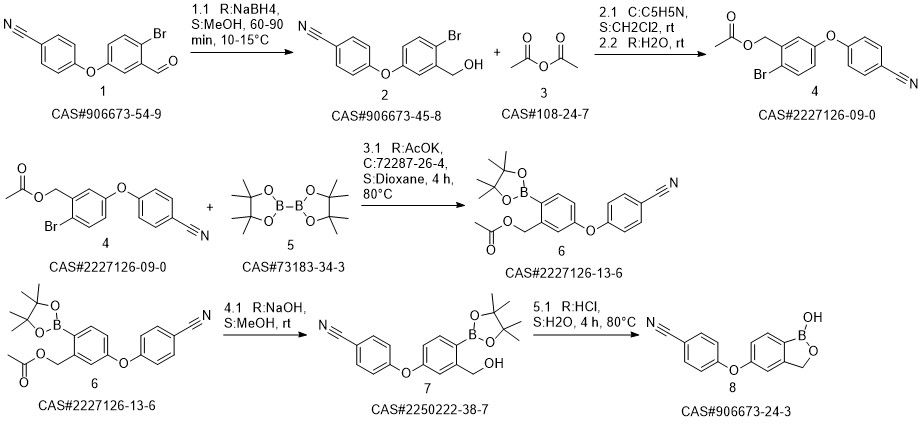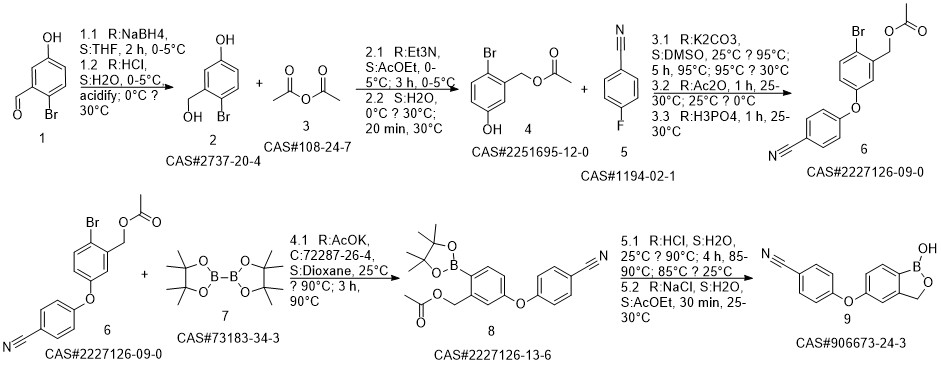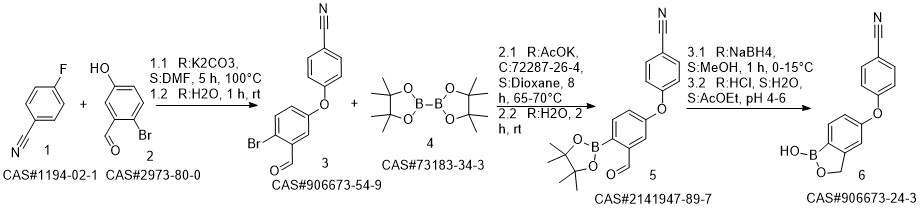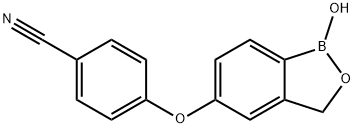
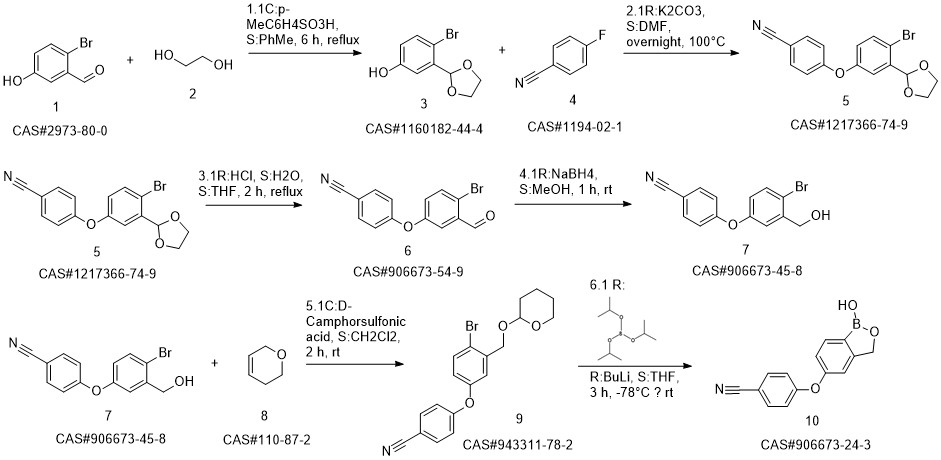
Crisaborole synthesis
- Product Name:Crisaborole
- CAS Number:906673-24-3
- Molecular formula:C14H10BNO3
- Molecular Weight:251.05
Reference: Akama T, Baker SJ, Zhang YK, Hernandez V, Zhou H, Sanders V, Freund Y, Kimura R, Maples KR, Plattner JJ. Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg Med Chem Lett. 2009 Apr 15;19(8):2129-32. doi: 10.1016/j.bmcl.2009.03.007. Epub 2009 Mar 9. PubMed PMID: 19303290.
![benzo[c][1,2]oxaborole-1,5(3H)-diol](/CAS/20200119/GIF/1187190-70-0.gif)
1187190-70-0
58 suppliers
$165.00/250MG
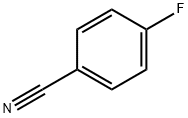
1194-02-1
517 suppliers
$6.00/10g

906673-24-3
430 suppliers
$45.00/10mg
Yield:906673-24-3 95%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 110; for 12 h;Reagent/catalyst;Temperature;
Steps:
1.6 Synthesis of S6 and Kleblon:
Add to the round bottom flask with mechanical stirringA 161g nitrile fluorophenyl,2-hydroxymethyl-5-hydroxybenzeneboronic acid half ester 200g,Potassium carbonate 221g,N,N-dimethylformamide 2000mL,Raise the temperature to 110 ° C for 12 h,HPLC monitors the progress of the reaction,After the raw materials are completely converted,Filter to remove solids,The filtrate was poured into ice water and quenched.Extracted with ethyl acetate,Dry with anhydrous sodium sulfate,Concentrated to a crude product of gramboroline,Then beat with n-hexane, filter,Dry the gram of Bora.
References:
CN109456347,2019,A Location in patent:Paragraph 0052; 0059; 0067; 0075; 0077
![benzo[c][1,2]oxaborole-1,5(3H)-diol](/CAS/20200119/GIF/1187190-70-0.gif)
1187190-70-0
58 suppliers
$165.00/250MG
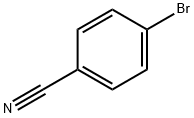
623-00-7
486 suppliers
$6.00/10g

906673-24-3
430 suppliers
$45.00/10mg
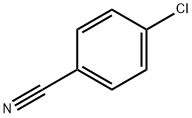
623-03-0
474 suppliers
$6.00/25g
![benzo[c][1,2]oxaborole-1,5(3H)-diol](/CAS/20200119/GIF/1187190-70-0.gif)
1187190-70-0
58 suppliers
$165.00/250MG

906673-24-3
430 suppliers
$45.00/10mg