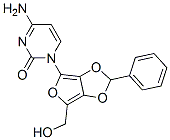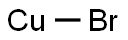
Cuprous bromide synthesis
- Product Name:Cuprous bromide
- CAS Number:7787-70-4
- Molecular formula:BrCu
- Molecular Weight:143.45
7758-99-8
569 suppliers
$6.00/25g
7647-15-6
879 suppliers
$12.50/100G
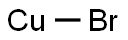
7787-70-4
452 suppliers
$6.00/25g
Yield:-
Reaction Conditions:
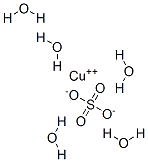
Stage #1: copper(II) sulfate;sodium bromide in water;
Stage #2: with sodium sulfite in water;
Stage #3: with hydrogen bromide in hexane;
Steps:
28
Example 2828.1 28.2[0329] Methyl 4-bromo-3-(trifluoromethoxy)benzoate (28.2). To a solution of 4-amino-3-(trifluoromethoxy)benzoic acid (2.00 g, 9.10 mmol) in MeOH (25.0 mL), was slowly added HCl (1.0 mL, 1.0 M in ether) at room temperature. The resulting reaction mixture was stirred at room temperature overnight. Benzene (20 mL) was added, and the reaction was heated at reflux with a Dean-Stark trap to remove the half volume of the solvent. The rest of the solvent was then evaporated to give the product. MS (ESI) m/e = 235.9 [M+l]+, Calc'd for CgHeF3NOs, 235.1. The crude product was used in the next step without further purification. To an ice-cooled suspension of methyl 4-amino-3- (trifluoromethoxy)benzoate hydrogen chloride salt (8.60 g, 31.70 mmol) in 17.1 mL of water and concentrated HBr (48 %, 17.1 mL), was slowly added a prepared 2.5 M solution of sodium nitrite (2.20 g in 12.7 mL) at 00C. The reaction mixture
References:
WO2008/30520,2008,A1 Location in patent:Page/Page column 136
7789-45-9
385 suppliers
$6.00/25g
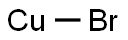
7787-70-4
452 suppliers
$6.00/25g