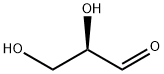
D-Glyceraldehyde synthesis
- Product Name:D-Glyceraldehyde
- CAS Number:453-17-8
- Molecular formula:C3H6O3
- Molecular Weight:90.08
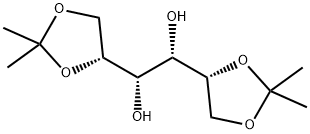
1707-77-3
359 suppliers
$5.00/5g

453-17-8
112 suppliers
$25.00/100mg
Yield:453-17-8 18 g
Reaction Conditions:
with sodium periodate;sodium hydrogencarbonate in dichloromethane at 0 - 20; for 5 h;
Steps:
1S)-1-[(2R)-1,4-Dioxaspiro[4.5]dec-2-yl]-3-buten-1-ol (5).
To a cooled (0 oC) solution of mannitol diacetonide(20 g, 58.47 mmol) in CH2Cl2 (100 mL), NaIO4 (25.02 g, 116.95 mmol) followed by sat. NaHCO3 (8 mL) wereadded and stirred at room temperature for 5 h. Reaction mixture was dried (Na2SO4), filtered and evaporatedunder reduced pressure to give (R)-Glyceraldehyde (18 g) and used as such to next reaction.To a stirred and cooled (0 oC) mixture of (R)-Glyceraldehyde (18 g, 105.26 mmol) and dry Zinc (13.7 g, 210.50mmol) in THF (100 mL), allyl bromide (10.7 mL, 126.30 mmol) was added very slowly for 15 min, followed bythe addition of sat. NH4Cl (72 mL) solution. After 6 h, reaction mixture was diluted with excess sat. NH4Clsolution (50 mL) and extracted with ethyl acetate (2 x 100 mL). The organic layers were washed with water (2x 20 mL), brine (20 mL), dried (Na2SO4), evaporated under reduced pressure and purified the residue bycolumn chromatography (Silica gel, 60-120 mesh, 5% EtOAc in pet. ether) to furnish 5 (17.5 g, 78%) as a yellowliquid. [a]D +1.7 (c 2.5, CHCl3); IR (neat): 2985, 2936, 2863, 1613, 1513 cm-1; 1H NMR (CDCl3, 500 MHz) δ (ppm):5.87-5.77 (m, 2H), 5.14-5.10 (m, 2H), 3.98-3.91 (m, 2H), 3.89-3.85 (m, 1H), 3.72-3.69 (m, 1H), 2.23-2.14 (m,2H), 1.60-1.55 (m, 8H), 1.41-1.38 (m, 2H); ESIMS: 235 (M+ Na)+, 213 (M + H).
References:
Bommagani, Shobanbabu;Thodupunuri, Prashanth;Sharma, Gangavaram V.M. [Arkivoc,2017,vol. 2017,# 4,p. 20 - 33]
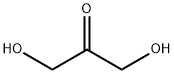
96-26-4
497 suppliers
$9.00/10g

453-17-8
112 suppliers
$25.00/100mg
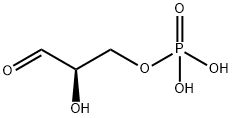
591-57-1
14 suppliers
$316.00/1ml

453-17-8
112 suppliers
$25.00/100mg
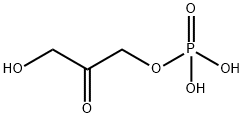
57-04-5
19 suppliers
$65.00/25 mg
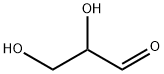
56-82-6
173 suppliers
$68.90/500mg

453-17-8
112 suppliers
$25.00/100mg
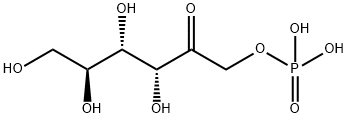
19456-80-5
0 suppliers
inquiry
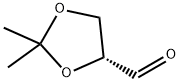
15186-48-8
322 suppliers
$8.00/5g

453-17-8
112 suppliers
$25.00/100mg