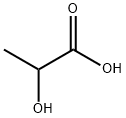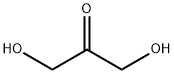
1,3-Dihydroxyacetone synthesis
- Product Name:1,3-Dihydroxyacetone
- CAS Number:96-26-4
- Molecular formula:C3H6O3
- Molecular Weight:90.08

56-81-5
1771 suppliers
$5.00/25g

96-26-4
497 suppliers
$9.00/10g
Yield:96-26-4 95%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical in water;acetonitrile at 60; under 760.051 Torr; for 12 h;
Steps:
1
Glycerol (1.0 mmol) and TEMPO (2.0 mmol) were placed in a flask under an air atmosphere (1 atm) and dissolved by adding a mixed solvent of 1 mL of water and 3 mL of acetonitrile. Here, 50 mg (containing Cu 2 +: 4.5 mg) of "Cu (II) -AlO (OH)" obtained in Preparation Example 1 was added and the reaction was carried out at 60 ° C. for 12 hours. The reaction progresses to TLC (I2 coloration) I monitored. After completion of the reaction, the reaction solution was centrifuged to remove "Cu (II) -AlO (OH)" and the supernatant was passed through a silica gel column (acetone eluent) to remove TEMPO, And concentrated to isolate a pale yellow oily reaction product. HPLC of the reaction product, 1 H-NMR, and As a result of 13 C-NMR analysis, It was confirmed to be dihydroxyacetone, The raw material conversion was 100%, and the yield of dihydroxyacetone was 95%.
References:
DAICEL CORPORATION;OSAKA UNIVERSITY;KANEDA, KIYOTOMI;MATSUDA, HIROKAZU JP6099133, 2017, B2 Location in patent:Paragraph 0051
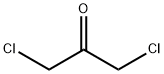
534-07-6
0 suppliers
$10.00/1g

96-26-4
497 suppliers
$9.00/10g
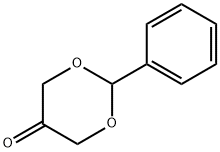
52941-82-9
31 suppliers
$262.00/1g

96-26-4
497 suppliers
$9.00/10g

56-81-5
1771 suppliers
$5.00/25g

96-26-4
497 suppliers
$9.00/10g
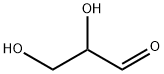
56-82-6
173 suppliers
$68.90/500mg
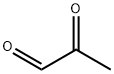
78-98-8
307 suppliers
$10.60/1gm: