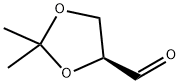
(S)-Glyceraldehyde acetonide synthesis
- Product Name:(S)-Glyceraldehyde acetonide
- CAS Number:22323-80-4
- Molecular formula:C6H10O3
- Molecular Weight:130.14
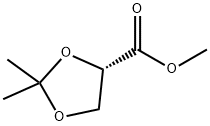
60456-21-5
106 suppliers
$40.00/1 / G

22323-80-4
143 suppliers
$71.50/5g
Yield:22323-80-4 89.9%
Reaction Conditions:
with diisobutylaluminium hydride in hexane at -78; for 5 h;
Steps:
1 Production of 2,3-O-isopropylidene-L-glyceraldehyde
Under an argon atmosphere, hexane (39 ml, 0.88M) was added to methyl 2,3-O-isopropylidene-L-glycerate (5 ml, 34.5 mmol), and the mixture was cooled to -78°C. After cooling, diisobutyl aluminum hydride (0.95M hexane solution) (40 ml, 38.0 mmol) was added, and the mixture was stirred at -78°C for 5 hr. After confirmation of completion of the reaction by TLC (hexane:ethyl acetate=2:1), aqueous ammonium chloride solution was added, and the temperature was allowed to return to room temperature. The reaction mixture was filtered and extracted with diethyl ether. The diethyl ether layer was dried over magnesium sulfate, and after filtration, concentrated under reduced pressure. Thereafter, the residue was purified by silica gel column chromatography (hexane:ethyl acetate=3:1) to give 2,3-O-isopropylidene-L-glyceraldehyde as a colorless transparent liquid (3.3 g, yield 89.9%). 1H-NMR (CDCl3, 400MHz)δ1.42(s, 3H), 1.49(s, 3H), 4.07-4.20(m, 2H), 4.40(dt, 1H, J=1.8Hz, J=7.0Hz), 9.72(d, 1H, J=1.8Hz)
References:
Ajinomoto Co., Inc. EP1574515, 2005, A2 Location in patent:Page/Page column 16
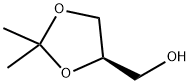
14347-78-5
359 suppliers
$6.00/1g

22323-80-4
143 suppliers
$71.50/5g
![1,2-Ethanediol, 1-[(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]-, (1S)-](/CAS/20200611/GIF/91274-05-4.gif)
91274-05-4
0 suppliers
inquiry

22323-80-4
143 suppliers
$71.50/5g
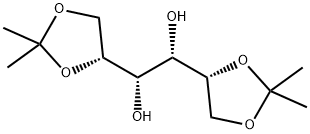
1707-77-3
359 suppliers
$5.00/5g

22323-80-4
143 suppliers
$71.50/5g
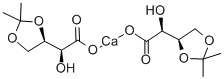
98733-24-5
9 suppliers
$39.70/1g

22323-80-4
143 suppliers
$71.50/5g