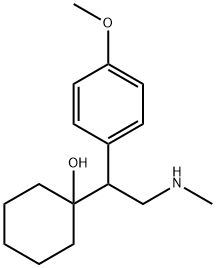
D,L N-Desmethylvenlafaxine synthesis
- Product Name:D,L N-Desmethylvenlafaxine
- CAS Number:149289-30-5
- Molecular formula:C16H25NO2
- Molecular Weight:263.38

272788-07-5
17 suppliers
$315.00/10MG

149289-30-5
42 suppliers
$198.00/5mg
Yield: 88.8%
Reaction Conditions:
with dimethylsulfide borane complex in tetrahydrofuran at 0 - 20; for 5 h;Reflux;
Steps:
3
To a solution of crude N-formyl compound (0.585 g, 2.1 mmol) in 6 mL THF was added BH3.Me2S (0.480 g, 0.63 mL of 10 M solution, 6.3 mmol) slowly at 0° C. The reaction was allowed to warm to room temperature and then was refluxed for 5 hours. It was cooled to 0° C. and 5 mL of methanol was added very carefully controlling the temperature below 10° C. The reaction was stirred for 10 minutes and volatiles were evaporated off. Residue was partitioned between 3N HCl (20 mL) and ethyl acetate (20 mL). Organic layer was separated and aqueous layer was extracted with ethyl acetate (3×15 mL). Aqueous layer was cooled to 0° C. and was basified by slow addition of conc. NH4OH. Aqueous layer was saturated with NaCl and was extracted with ethyl acetate (3×20 mL). Combined ethyl acetate layer was dried (Na2SO4), ethyl acetate was evaporated in vacuo to give colorless oil (0.493 g, 88.8% yield). (CDCl3): 7.15 and 6.85 (q, 4H), 3.80 (s, 3H), 3.25 (dd, 1H), 2.95 (dd, 1H), 2.82 (dd, 1H), 2.45 (s, 3H), 1.40 (m, 10H); 13C (CDCl3): 158.4, 133.0, 130.5, 113.7, 73.9, 55.4, 53.8, 53.0, 37.8, 36.5, 33.7, 26.0, 21.9.
References:
Sunovion Pharmaceuticals Inc.;Jerussi, Thomas P.;Senanayake, Chrisantha H.;Bhongle, Nandkumar N. US2015/299102, 2015, A1 Location in patent:Paragraph 0097
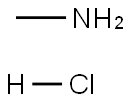
593-51-1
531 suppliers
$21.00/100g
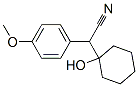
93413-76-4
246 suppliers
$22.00/100mg

149289-30-5
42 suppliers
$198.00/5mg
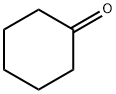
108-94-1
527 suppliers
$12.00/50g

149289-30-5
42 suppliers
$198.00/5mg

93413-76-4
246 suppliers
$22.00/100mg

149289-30-5
42 suppliers
$198.00/5mg
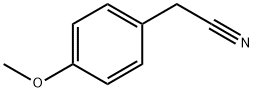
104-47-2
460 suppliers
$6.00/10g

149289-30-5
42 suppliers
$198.00/5mg